feynmann
Harmless

Posts: 29
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
some details needed
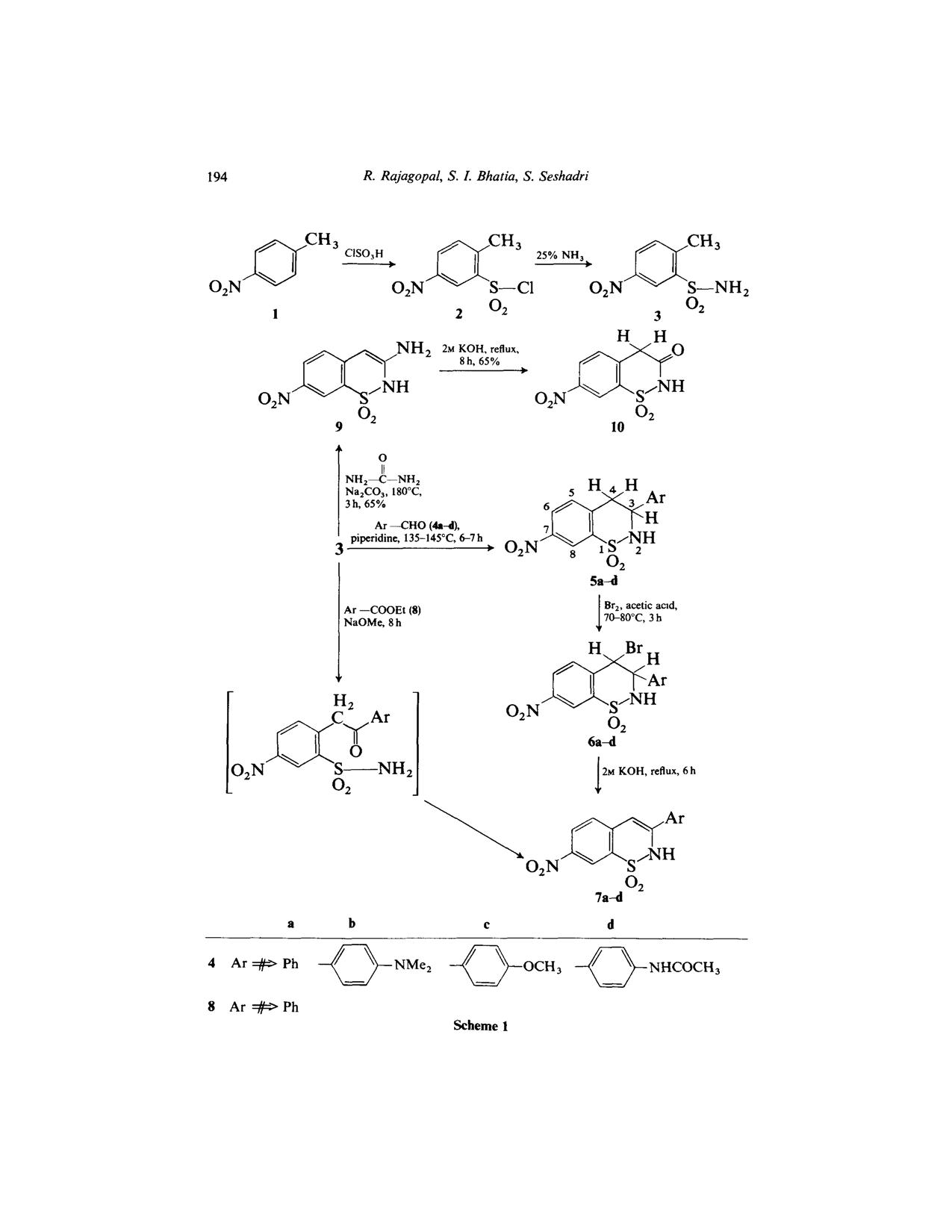
I want to know the supposed condition for this reaction if u please??
Reacting ester 8 with 3 using sodium methoxide ........what is the solvent used ,,,IS it methanol and what is the supposed temperature to be used
???(I don't think he didn't use a solvent as sodium methoxide has auto ignition point of 88 degree C
The picture of scheme is below and the paper is posted in the next post or u can download here
http://rapidshare.com/files/134990933/sdarticle.pdf.html
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
If you have a look in the experimental you will see that the substrates were heated with the neat sodium methoxide (page 199).
|
|
|
feynmann
Harmless

Posts: 29
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
here is the article
Attachment: sdarticle.pdf (557kB)
This file has been downloaded 724 times
|
|
|
feynmann
Harmless

Posts: 29
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
I read it
For the synthesis of 3-aryl-7-nitro-2H-l,2-benzothiazine-l,l-dioxide (7a) a
mixture of 3 (1.76 g, 10 mmol), 8 (O-15 g, 10.1 mmol) and sodium methoxide
(1.5 g) was heated for 8 h. The brown reaction mass was run into ice-cold
water (15 ml) and then neutralised with dilute hydrochloric acid. The
product was crystallised from dimethylformamide. Reaction conditions,
yields, physical and spectroscopic data are given in Table 1.
It doesn't mention the temperature and that what I wanted to know
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I would guess that the mixture was heated until it melted and the melt was then stirred.
The reaction temperature would then depend on the substrates being used to a certain extent.
It does mention running the reaction mixture into ice water so I would guess it is molten at the end of the reaction.
You could write to the authors.
[Edited on 18-8-2008 by ScienceSquirrel]
|
|
|
feynmann
Harmless

Posts: 29
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
Heating sodium methoxide without solvent is strange to me somehow as it can ignite so easily and it decomposes before melting too
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I would heat it under nitrogen to avoid the catching fire / oxidation of the substrate bit etc.
Also the reaction eliminates water so I think you probably end up with an oily mixture when the reaction is done.
Anyway I would heat it in a small flask under a condenser and N2 atmosphere until it melted and leave it in the overnight room and head off for my pie
and chips.
See how the stuff was looking in the morning 
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
It's quite likely in methanol.
|
|
|
feynmann
Harmless

Posts: 29
Registered: 6-8-2008
Member Is Offline
Mood: No Mood
|
|
To science squirrel I point also that sodium methoxide may decompose on direct heating
Did u meet any procedure with fusion of sodium methoxide before??
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I don't think that they are fusing the sodium methoxide, I think it is is dissolving in the molten reactants.
The fact that DMF was chosen as a recrystallisation solvent suggests that the product is not overly soluble in most solvents.
Most experienced chemists would avoid DMF like the plague as a recrystallisation solvent as it is a right pain to get rid of.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by feynmann
To science squirrel I point also that sodium methoxide may decompose on direct heating
Did u meet any procedure with fusion of sodium methoxide before?? |
Sodium methoxide does not decompose so easily. It can catch fire if heated in the presence of air, but nobody is saying you can not use inert
atmosphere. If the experimental does not say at which temperature the reaction mixture was heated, then follow with TLC like you are supposed to
anyway.
PS: Please do not use pictures with such large width. It will deform the frame set of many members who use the most common monitor resolutions. Also,
this forum is supposed to be about amateur chemistry and science and posting only questions related to your job is not particularly welcome
unless you are involved in other discussions as well. There already exist a number of forums for professional chemists where you can discuss job
related questions like this.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I am just giving my opinion on the basis of my experience and the sparse details in the paper. I am a computer bod these days.
I cannot remember the exact conditions but I remember using sodium methoxide in hot DMF to convert bromoarenes to aryl methoxyethers so as Nicodem
says it will certainly take some welly before breaking down.
|
|
|