Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Synthesis of 4-(3,4-methylendioxyphenyl)butan-2-one, "Cassione"
This coumpound is said to posses the aroma of black currant. There is little information on this compound on the net.
From US 5908770:
| Quote: | "Cassione.RTM (registered trade mark of the company
Firmenich).", or 4-(3,4-methylenedioxyphenyl)-butan-2-one, had not been
found in nature until recently. For this reason, outside perfumery, its
use had only been authorised in artificial food flavourings. Its presence
was recently established in the essential oil of the aerial part of the
rue plant: Ruta angustifolia Pers., a plant which is self-sown and grows
in Malaysia (D. Joulain et al., Journal of Essential Oil Research, 1991,
3, 355), and it is therefore possible to now use this substance in food
flavourings labelled "natural". |
Process for preparing butanone derivatives
Being a 4-phenyl-2-butanone, like zingerone ( 4-(4-hydroxy-3-methoxyphenyl)butan-2-one ) and rheosmin ( 4-(4-hydroxyphenyl)butan-2-one, the first idea
that comes to mind is to follow the same path as for the two others, condensation of the aldehdye with acetone, and reduction of the unsaturated
ketone.
But to avoid preparing the controversial aldehdye piperonal, and because the benzylic alcohol is a starting material much more accesible, I decided
on following another route, namely condensation of the benzyl halide with acetoacetic ester, followed by ketonic hydrolysis and decarboxylation to the
butanone.
Converting the benzylic alcohol to the bromide, and performing the well-known acetoacetic condensation seems like a shorter and more efficient
alternative to oxidizing the the alcohol to the aldehdye, and performing the aldol and reduction.
the condensation of 4-methoxybenzyl chloride with AcAcOEt in presence of K2CO3 and acetone is described in GB 1 094 417, although in moderate overall
yields. The more conventional EtONA in absolute EtOH would surely give much better yields, and IPA can surely be used with perhaps even better resuts
(the isopropoxide being a stronger base than EtONa), avoiding the use/preparation of absolute EtOH, aswell as in t-amyl alcohol and it's alkoxide
(JACS; 66(1), 144-146 (1944) )
The condensation can also be performed with NaH in aprotic solvents and PTC (J. Org. Chem.; 39(22), 3271 - 3273 (1974 ) ), or even by using
NaOH/KOH in dry alcohol (Acta Chem. Scand.; 13(3), 607-608 (1959)), although that last option might not be appliable to an activated benzyl bromide such as
piperonyl bromide. The salts of AcAcOEt can be prepared using toluene and a dean stark before alkylation (J. Org. Chem. 1961; 26(3) pp 644 - 651 ),
and the presence of DMF is said to be quite benefical, although it can promote O-alkylation with active alkyl halides. (most of the refs are availble
at the ref forum)
The preparation of the benzyl halide is discussed in this thread.
I tried preparing piperonyl bromide form the alcohol yesterday, using "in-situ" 40% HBr from KBr and H2SO4, and adding the solid piperonyl alcohol in
portion over 10min. The solid gradaully turned to a semi-solid blob, and to a light amber oil after two hours with frequent shaking (test tube scale).
Unfortuanly, there was still some alcohol left after extarction with DCM, washing with dilute NaHCO3 until neutral and brine, and the product refuses
to solidify after removal of the solvant and cooling in the fridge.
I think I will try again with a co-solvent (toluene), a bigger excess (used 1.5x this time) of distilled HBr azeotrope, as is done in a WIPO patent
mentionned in the piperonyl chloride thread.
I will surely try the alkylation using iPrONa/IPA (adding NaH to dry IPA), and then work my way down to using toluene and forming the salt with KOH
and a dean stark in an eventual second try.
This method may be adequate for derivatives that are easily prepared by halomethylation, rather than going for the aldehydes. The reported high yields
for a sensitive substrate such as benzodioxol are very encouraging, and the whole reaction scheme looks much more easy than the reduction of the
a,b-unsaturated ketones (if one doesn't have Pd/C or doesn't want to use it).
Again, the trick is to see if it can be used with phenolic substartes. I ahev seen some patents were hindred halomethyl phenols are used such a
alkylation, but the phenols are generally surrounded by large groups. Things like p-hydroxybenzyl halide look pretty easily polymerized too. But it
could be worth a try.
[Edited on 5-10-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
I thought that you got buten -3- one from those reactions. I would be very happy if I am wrong 
e3500 console login: root
bash-2.05#
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, it's butan-2-one starting from the methyl alpha from the carbonyl, and not the phenyl ring. Obtaining a butan-3-one from the methyl group
wouldn't be possible with an AcAcOEt ester condensation, as haloarenes can't react by SN2 with the AcAcOEt anion..
Here is a diagram to resume everything:
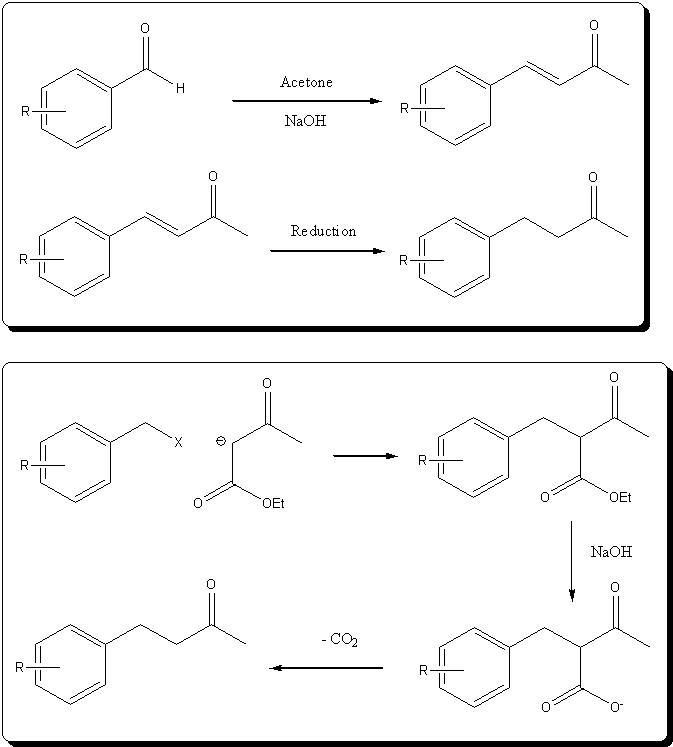
Sorry if I made you have false expectations 
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
So what about brominating or iodinating the starting aromatic, the doing a Heck reaction with MVK?
I thought of that, went to search for Heck using MVK, and one of the first hits was this
| Quote: | | The one-pot synthesis of the fragrance 4-(p-methoxyphenyl)butan-2-one, with raspberry scent, has been carried out using palladium on different
supports such as magnesium oxide (MgO), hydrotalcite, hydroxyapatite (HA), aluminium oxide (-Al2O3) and titanium dioxide (TiO2). The first pathway
consists of a Heck coupling between 4-methoxyiodoanisole and methyl vinyl ketone followed by hydrogenation. Palladium supported on titanium dioxide
showed the best performance for carrying out both consecutive steps giving 4-(p-methoxyphenyl)butan-2-one with high yields and selectivity. The
Pd-TiO2 catalyst is more active than a homogeneous palladium complex that is well accepted in the literature as being highly active for performing
Heck reactions. |
http://www3.interscience.wiley.com/journal/116307690/abstrac...
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
The heck reaction suffers from the same problem as the aldol; you need to reduce an a,b-unsaturated ketone to get the desired product.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
You can couple MVK direct with phenol to Rheosmin, there are several articles on this in the original Raspberry ketone thread. The reaction is
catalyzed by acids, Amberlysts, and soem zeolites, but MVK is pretty nasty.
But I've stumbled on a nice article where acetoacetic ester is alkylated with the mannich base of acetone or MEK, and it is assumed the mannich base
actually forms MVK in-situ, which is the true alkylating agents, via a Michael addition. Mayde the mannich base of butanone, formaldehdye and Me2NH
can be used, forming the MVK in a closed setup and directly bubbling it into the phenol in solution with a acidic catalyst? MVK is pretty volatil
IIRC. The salt of the mannich base will not react in acidic conditions though I think.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
thanx klute that's what kind I thought
e3500 console login: root
bash-2.05#
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Just found another interestinf alternative: the butanones can be formed by using a diazotized aniline and MVK:
Reductive arylation of electron-deficient olefins: 4-(4-chlorophenyl)butan-2-one (lots of interesting ref cited)
Need to work on the preparation of MVK, apaprently easily made from acetone, formaldehdye and diethylammonium chloride:
http://www.springerlink.com/content/n8v0045551157n01/
Hopefully dimethylammonium chloride can be used. I will look into this.
EDIT:
Another path, very promising. It actually looks even simplier than AcAcOEt alkylation: alkylation of acetylacetone in EtOH/K2CO3, the diketone is
directly cleaved into the butanone, and the authors report good yields for benzyl halide (>75%). AcAc is about as expensive as AcAcOEt, and the
alkylation doesn't require Na, NaH or simialr. The use of absolute ethanol can surely be substitued be dry IPA, or perhaps even acetone?
Any way, ithink I will try this before the AcAcOEt alkylation, as I'm pretty short on lab time at home, this would be more practical.
Synthesis of Ketones of the Type CH3COCH2R from Acetylacetone and Halides with Ethanolic Potassium Carbonate. An Alkylation-Cleavage
Process
Sandra Boatman, Thomas M. Harris and Charles R. Hauser
J. Org. Chem.; 30, 3321 (1965)
Abstract:
A number of ketones of the type CH3COCH2R, where R is alkyl, aralkyl, or a related group, were prepared in good yield from acetylacetone and
appropriate halides by means of ethanolic potassium carbonate. This alkylation-cleavage process appears more convenient than earlier procedures. Other
courses of reaction were observed with certain halides. One of these involved dialkylation and cleavage to give the ketone of the type CH3COCHR2; this
process was usefull when R was m-nitrobenzyl. Twofold monoalkylation and cleavage occured with a,a'-dichloro-p-xylene to form
4,4'-phenylenedi-2-butanone.
[Edited on 6-10-2008 by Klute]
Attachment: JOC_30_3321_1965_acac.pdf (548kB)
This file has been downloaded 1015 times
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I tried the preparation of piperonyl bromide this weekend, following the procedure from WO 98/22417 (see the piperonyl chloride thread for the pdf),
using toluene instead of benzene.
20 mmol of piperonyl alcohol were added in portions to a stirred mixture of 15mL 48% HBr and 55mL toluene, cooled to 5°C. The white solid dissolved
only slowly, and the temp was left to increase to 10-15°C. Even after 3h, there was still some alcohol left by TLC (40:15 Pet ether: AcOEt), so a
spatula tip of NBu4.HSO4 was added. After another 30min, the alcohol was still there, so the two layers were seperated, the aqueous extracted with
3x5mL toluene, the combined extracts washed with 10mL water, 2x20mL 5% sodium bicarbonate solution, and brine. After drying overnight over Na2SO4,
most o fthe toluene was removed under vacuum, leaving ~5mL. The concentrated solution was kept in the fridge under argon awaiting to be used in the
AcAc condensation. It seemed another impurity had appeared after removal of the solvent (at 50-60°C), perhaps the acid from oxidation of the
remaining alcohol. It is hard to judge the amount of alcohol left, but the stain is only slightly smaller than the benzyl bromide stain.
I think I will try forming the chloride by gassing a toluene solution with dry HCl gas, and just extended the reflux period during the alkylation, as
the benzyl chloride is surely pretty recative itself... Hopefully the amount of residual alcohol will be minimal or nile.
I will post a few pictures when I get time.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
|