Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
Oxidation of a phenolic derivative with ammonium persulfate
Hi, I found a reference to oxidation of secondary alcohols to produce ketones. The reaction vessel was charged with the following:
50ml Sesamol
100g Ammonium Persulfate
150ml Water
It involves keeping the solution at 85oc with stirring for one hour. I was happy to oblige, however I let it get a little too hot (95oc) whereupon a
runaway oxidation occurred (even with heat taken off). The whole thing was over in less than 3 minutes. Much of the water had boiled away.
Does this cause any side products to be formed? Or is it still going to produce mostly the desired product?
Further searches on this phenomenon have failed to yield any relevant information.
Edit by Chemoleo: Title as it was misleading
[Edited on 16-10-2008 by chemoleo]
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
I found something that is somewhat related but it discusses nitric acid as the oxidant:
http://www.sciencedirect.com/science?_ob=ArticleURL&_udi...
The thermal runaway behavior of an exothermic, heterogeneous, multiple reaction system has been studied in a cooled semi-batch reactor. The nitric
acid oxidation of 2-octanol has been used to this end. During this reaction, 2-octanone is formed, which can be further oxidized to unwanted
carboxylic acids. A dangerous situation may arise, when the transition of the reaction towards acids takes place accompanied by a temperature runaway.
An experimental set-up was build, containing a 1-l glass reactor, followed by a thermal characterization of the equipment. The operation conditions,
e.g. dosing time and coolant temperature, to achieve a high yield under safe conditions are studied and discussed. The reaction conditions should
rapidly lead to the maximum yield of intermediate product 2-octanone under safe conditions and stopped at the optimum reaction time. The appropriate
moment in time to stop the reaction can be determined by model calculations. Also, operation conditions are found, which can be regarded as invariably
safe. In that case, no runaway reaction will occur for any coolant temperature and the reactor temperature will always be maintained between
well-known limits. The boundary diagram of Steensma and Westerterp [1990] for single reactions can be used to determine the dosing time and coolant
temperature required for safe execution of the desired reaction. For suppression of the undesired reaction, it led to too optimistic coolant
temperatures.
The reason I question it's applicability is that as far as I know persulfate does not generate carboxylic acids from ketones. I might need to do a
ketone test on the resulting mixture but of course that is qualitative rather than quantitative.
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Sesamol is not a secondary alcohol! It is a phenol! You can not make a ketone out of it. Utmost it can be made into benzoquinones - which are the
closest thing to ketones you can get from phenols.
| Quote: | Originally posted by Baphomet
Hi, I found a reference to oxidation of secondary alcohols to produce ketones. The reaction vessel was charged with the following:
50ml Sesamol
100g Ammonium Persulfate
150ml Water |
Are you trying to convince us that you run a reaction using 100EUR worth of sesamol just to destroy it? Is this some kind of a joke. 
And where is that reference you talk about?
The oxidation of alcohols with persulfates was discussed in length in a dedicated thread. Magpie made a series of experiments using this method,
including experiments in its up scaling. Read it.
| Quote: | | Does this cause any side products to be formed? Or is it still going to produce mostly the desired product? |
You mix an organic compound on a 0.36 mol scale (!) with an oxidant using radical chain oxidation mechanism and then act surprised
about the runaway reaction? And then you ask if it is still going to produce the fictional desired product?
You must be making fun of us. 
[Edited on 15/10/2008 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
Nico, try some breathing exercises. You seem stressed out.
Sesamol is the aromatic form of a secondary alcohol. In other words it is an phenolic compound with the hydroxyl attached directly to the ring. It's
not a difficult concept. 
Benzoquinone and related compounds are aromatic forms of ketones. It is quite acceptable for the oxygen to connect directly to the ring. 
My reference for the scheme was:
"Oxidation of alcohols with ammonium persulfate"
Hassan et. al.
Chinese Journal of Chemistry, 2007 Vol. 25 No. 6
This same ref uses the procedure described to form cyclohexanone.
The reaction scheme described by the authors does not rely on R1, R2 being part of a straight chain. Indeed it does not even rely on the compound
being saturated. So it is very selective to the hydroxyl function compared to some other oxidation methods.
Oh yeah, and 100 bucks is not that much money 
In short I want to know if this reaction would still work. Some of the oxidations we did at uni involved violent reactions and yet still produced good
yield of the target product. One should not always assume that a runaway is automatic failure.
[Edited on 15-10-2008 by Baphomet]
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You seriously bleached 50g of sesamol just for the fun of it? Goddamn, that thing is so expensive and I have been looking for it for years. No lab
around here has any and I surely don't want to pay it out of my pocket. And you just go there destroying it like it's nothing! 
Anyway, phenols can not be considered secondary alcohols for the simple reason since the OH group is bound on an sp2 carbon. In short, phenols can
(only formally) be considered either "aromatic alcohols" or "enolized ketones". Since they formally already are ketones (at least by their oxidation
state) they can not be oxidized to ketones. However, the aromatic system can be oxidized to a quinone system - this has nothing to do with alcohols
oxidation since it is about the Pi system oxidation (in theory you can oxidise even benzene to benzoquinone). In short, if you your "desired product"
is 4,5-(methylenedioxy)-o-benzoquinone then you could have used any of the literature method. For example, oxidation of sesamol with Fremy's
salt (Justus Liebigs Annalen der Chemie, 719 (1969) 112-118 or Tetrahedron, 60 (2004) 8899-8912).
Next time do some reading before wasting precious chemicals and putting yourself in danger of injury from a runaway reaction. Do you have some
suicidal tendencies to try out persulfate reactions on such scale before even checking on a mmol scale? Do you think the authors of that paper made
the reactions on a mmol scale just to appear fancy?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
ketones are not quinones and quinones are not ketones.
just as phenols are not alcohols.
Nicodem very rarely is upset by posts.
and 99% of the time he is more than accurate.
as for runaway reactions not being a failure. hahaha did you say university in the same post.
you have a death wish and information that leads to runaway reactions could hurt others.
no wonder he was not his normal pleasant self.
a note on sesamol. you oxidized it. you should be shot.
get a copy of shulgins work and beat your self in penance.
[Edited on 15-10-2008 by Ephoton]
e3500 console login: root
bash-2.05#
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
Nicodem: Nothing in my experiment was created or destroyed. Your anger is irrational to say the least. 
So you are conceding that I'm right. This "only formally" stuff is just a semantic ploy. The fact remains that delocalisation of charge in the ring
only has so much influence, for a lot of intents and purposes the substance in question acts like an alcohol.
Ephoton: Danger, give me a break! We are not talking about TNT here. Learn a little more about which reactions are dangerous and which aren't, before
shooting your mouth off.
Actually I did go to uni. Did you?  Many times we conducted Jones' oxidation of
all kinds of alcohols in water and/or acetone. It was usually a violent reaction but no-one went to hospital and we always got the product we were
after. The only difference was that we introduced the feedstock slowly to prevent bubbling-over and such. Many times we conducted Jones' oxidation of
all kinds of alcohols in water and/or acetone. It was usually a violent reaction but no-one went to hospital and we always got the product we were
after. The only difference was that we introduced the feedstock slowly to prevent bubbling-over and such.
These were aliphatic so MAYBE in the case of sesamol I have cleaved the methylenedioxy or some other undesirable outcome. I don't know yet. But if you
don't know and don't have any suggestions then stop wasting my time with brainless insults.
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Baphomet
Nicodem: Nothing in my experiment was created or destroyed. Your anger is irrational to say the least.  |
So, you were really fooling us that you actually did something as stupid as burning 50g sesamol? That was not funny!
| Quote: | | So you are conceding that I'm right. This "only formally" stuff is just a semantic ploy. The fact remains that delocalisation of charge in the ring
only has so much influence, for a lot of intents and purposes the substance in question acts like an alcohol. |
I just explained you the oposite. That phenols are not secondary alcohols and that they can not be oxidized into ketones because they already are
"ketones" according to their oxidation state. At least do some reading before making such absurd claims. "Although similar to alcohols, phenols have
unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom)." (cf. http://en.wikipedia.org/wiki/Phenols). This is anything but semantics - it is about chemical properties and chemical structure, not about what you
want to call them!
And don't bullshit us about your university degree. No chemists with a degree would ever call sesamol or any other phenol a secondary alcohol. That is
as ignorant as calling a carboxylic acid a primary alcohol because it has a hydroxyl group (or calling amides for amines, etc.). Such misunderstanding
can only come from someone who never read much about organic chemistry and does not even care to do a literature search before doing a lab experiment.
Obviously you don't know much about what you are doing since you compared a Jones oxidation with a persulfate oxidation. A persulfate oxidation is a
self propagating radical oxidation which when scaled up can results in flasks blowing up. Please do not attempt any reactions above mmol scale,
fictional or real, before you learn the basics and master same practical skills. The last thing I want to hear is about our forum member's obituaries
in the news.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Hot persulfate is a very powerfull oxidant. The kind that turns any organic compound into carbon and other crap. There is a decomposition temp, a buit
over 70°C, where it violent decomposes. You get cleavage of any ethers, hydroxylation of rings, followed by all kind of side reaction possible with
SEM etc. Niot much will survive that.
I don't think you arre really well placed to suggest others to go and learn how dangerosu reactions are. USing such a powerfull oxifdant at such a
scale could have got you seriously hurt. Also, one of the most logical things that a chemists learns is that when trying out a new reaction,
especially with reactive reagents, and expensive substrates, yous tart low until you find the good conditions, and then work up to a preparative
scale.
Try learning more about how differentphenols and alcohol are, and what oxidation products one can obtain from phenols. Simialrily, aniline and
cyclohexylamine are not at all simialr in ways of reactivity..
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
A phenol can be oxidised to a quinone, simple as that!
You are mixed up. Jones oxidation is similar, did you ever see it? I'm not talking textbooks here but real life.
The flask did not blow up. I am here to prove that. The way you talk about this is so over the top.. as though I am working with pyridine complexes of
Cr. There is no feasible way for the reaction described in this thread to turn explosive.
Yes I have a uni degree. I never said I majored in chem.
This is not bullshit, it is real life. I'm not a millimole kind of guy. Where do you get off criticising me? I will use kilograms of reagent if I
want. It's none of your business!   
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Let's keep this civil.
It doesn't matter what degree anyone has.
What matters is facts.
The OH on phenols cannot be readily oxidised without breaking the original ring resonance bonding. In a more complex structure like sesamol I wouldn't
like to wager a guess as to its oxidation products.
Nonetheless I'd like request the following, Baphomet, since you are proposing this:
Structure of Sesamol

Now draw out your proposed mechanism of oxidation, to whatever product you think you'd get? Use Chemdraw, Isisdraw, whatever.
I'd like to see that proposed quinone.
Perhaps we can then trouble-shoot where precisely you are going wrong.
[Edited on 16-10-2008 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
| Quote: | Originally posted by Baphomet
I found something that is somewhat related but it discusses nitric acid as the oxidant:
http://www.sciencedirect.com/science?_ob=ArticleURL&_udi...
The thermal runaway behavior of an exothermic, heterogeneous, multiple reaction system has been studied in a cooled semi-batch reactor. The nitric
acid oxidation of 2-octanol has been used to this end. During this reaction, 2-octanone is formed, which can be further oxidized to unwanted
carboxylic acids. A dangerous situation may arise, when the transition of the reaction towards acids takes place accompanied by a temperature runaway.
An experimental set-up was build, containing a 1-l glass reactor, followed by a thermal characterization of the equipment. The operation conditions,
e.g. dosing time and coolant temperature, to achieve a high yield under safe conditions are studied and discussed. The reaction conditions should
rapidly lead to the maximum yield of intermediate product 2-octanone under safe conditions and stopped at the optimum reaction time. The appropriate
moment in time to stop the reaction can be determined by model calculations. Also, operation conditions are found, which can be regarded as invariably
safe. In that case, no runaway reaction will occur for any coolant temperature and the reactor temperature will always be maintained between
well-known limits. The boundary diagram of Steensma and Westerterp [1990] for single reactions can be used to determine the dosing time and coolant
temperature required for safe execution of the desired reaction. For suppression of the undesired reaction, it led to too optimistic coolant
temperatures.
The reason I question it's applicability is that as far as I know persulfate does not generate carboxylic acids from ketones. I might need to do a
ketone test on the resulting mixture but of course that is qualitative rather than quantitative. |
I would think this as an excellent starting point if you are interested in runaway kinetics and experimental design, if you buy it make sure you post
it i would be interested from a experimental design point of view. It's of less relevance the actual system but rather how they studied a tricky
system. Notice however they with all their careful consideration, they only designed a 1L reactor will full cooling, indicating that you don't need
much for things to spray up out of your 1l toluene/h2so4/persulfate in a 5L beaker (hmm). i cleaned up that shit from my ceiling walls floor for
days, at least it smelt good, i did however have to step backwards rather quickly at one stage. I managed to control it 1 of 3 times oxidising only
20mL or toluene in an open stirrred beaker, the other times it went 'bahh'. it's too sensitive for anything bigger than mmol.
However can i make a suggestion, get like 200ml of sesamol and video yourself pouring it down the drain, then post it, now that would be a funny
thread!
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
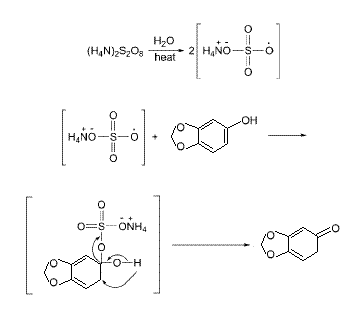
Chemoleo, here it is. It is my rough idea of what is happening.
Maybe the ring is broken but I don't think so. If something went wrong it is more likely that it was oxidised in more than one place (two hydroxyls on
the ring? two keto groups?)
This is because I did not see any char. The result was crystalline and brown but not black or burnt. No polymerisation either.
Panache, thanks for the info. Interesting. One has to wonder why the authors of the original paper said that this method is a viable alternative to
other methods... when scaling up results in something like this. Perhaps if the temp was controlled more precisely but even then that is no guarantee.
Anyway. I find I can hardly make a post around here these days without being flamed for no reason. Maybe it's time to say 'goodbye' to SciMad and
'hello' to another board where people are more accepting of others ideas.
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I don't get it. You start asking questions, and when people try to rectify you you get all mad... Seems like you have all the answers you want. You
surely have a flask full of quinone then. But the oxidation product you showed is not a quinone... Any text book would have shown you that that
compound is far less favored than an aromatic ring.
Have you got anything to back up that reaction mechanism?
Never mentionned anything about explosions, but runaway reactions, which have been reported by several members with this oxidant. Trying a new
reaction with a powerfull oxidant is asking for trouble iMHO, but do what you want hey, mister megamole hardcore.
Have a look at the oxidation potentials:
S2O8 -2 + 2H+ + 2e- ——> 2HSO4- E = 2.12V
H2O2 + 2H+ + 2e- ——> 2H2O E = 1.77V
HSO5- + 2H+ + 2e- ——> HSO4- + H2O E = 1.44V
Peroxodisulfates are stronger oxidants that either H2O2 or Caro Acid, that will turn wood into carbon in a matter of seconds. I don't want to see what
that does on a face.
More examples:
| Quote: | COMMENTS: PRECAUTIONARY STATEMENT: Use of persulfates in chemical reactions
requires appropriate precautions and design considerations for pressure and thermal relief.
Decomposing persulfates will evolve large volumes of gas and/or vapor, can accelerate exponentially with
heat generation, and create significant and hazardous pressures if contained and not properly controlled or
mitigated.
Use with alcohols in the presence of water has been demonstrated to generate conditions that require
rigorous adherence to process safety methods and standards to prevent escalation to an uncontrolled
reaction. |
http://www.hillbrothers.com/msds/pdf/n/ammonium-persulfate.p...
| Quote: |
Stability:
Unstable. Gradually decomposes losing oxygen. Decomposes more rapidly at higher temperatures. Stability decreases in the presence of moisture. Metals
other than stainless steel are apt to cause decomposition of persulfate solutions. |
http://www.jtbaker.com/msds/englishhtml/S4730.htm
| Quote: | Decomposition Hazard
Overheating or contamination of persulfates can lead to
a runaway decomposition. The persulfate salt will begin
to effervesce with an acid-like odor. Persulfates decompose
to form solid sulfate salts and emit noxious fog or
fumes of SOx and NOx. This decomposition may form a
high temperature melt. The material will flow like magma
and may ignite nearby combustible materials
such as wood or paper. Oxygen produced by persulfate
decomposition can increase the intensity of the fire. |
http://www.fmcchemicals.com/LinkClick.aspx?fileticket=y%2F0D...
Suit yourself.... Only tried to help you keeping your eyes, face and reagents...
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
| Quote: | Originally posted by Baphomet
Chemoleo, here it is. It is my rough idea of what is happening.
Maybe the ring is broken but I don't think so. If something went wrong it is more likely that it was oxidised in more than one place (two hydroxyls on
the ring? two keto groups?)
|
But Baphomet, this is not an oxidation, what you are showing is keto-enol tautomerisation!
http://en.wikipedia.org/wiki/Keto-enol_tautomerism
You are reducing a carbon double bond, while oxidising the OH- so in your proposed mechanism the role of NH4 persulfate would be that of a catalyst.
However, this is not a regular double bond, it is delocalised over the ring, and v. stable. Likely too stable to break up and accept a proton from the
solvent....
| Quote: | | This is because I did not see any char. The result was crystalline and brown but not black or burnt. No polymerisation either. |
This is interesting nonetheless. But you'd have to analyse some more to get an idea of what it is...
[Edited on 16-10-2008 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
I have no problem with people answering my questions. It is when they are condescending and rude that I take issue. Some of the comments made by
members here would result in a smack around the face if they said it to me in person.
"Obviously you don't know much about what you are doing.." < this kind of comment is not acceptable.
Basically I have started ignoring that guy. He seems a lot more interested in calling other people names and telling them that they are stupid, than
discussing chemistry.
Also, my threads are edited all the time. Meanwhile there are posts where the user cannot even spell the names of simple chemicals, and no-one
corrects them on that!
[Edited on 17-10-2008 by Baphomet]
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
Thanks chemeleo. That makes sense. Especially in light of the fact that the persulfate radical wants a proton, right?
I still wonder, for an actual oxidation.. of a straight-chain secondary alcohol, what kind of effect a very quick reaction would have. I will need to
try on 2-butanol or similar, and see.
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
| Quote: | Originally posted by Klute
.....mister megamole hardcore.
..... Only tried to help you keeping your eyes, face and reagents... |
Ha! Its just funny stuff, sry completely off topic (but i'm going to plagarise that endlessly)
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
What can I say? Some people get mole envy.
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
About mole envy,
I understand where you are coming from, being annoyed with mmol scale reactions and the like. I have been frustrated with this fact too, in the past;
with time though, I have learned that you save money, reagents and most importantly your priceless time by first conducting a reaction on a small
scale, working out the kinks and then scaling it up. I have learned that experimentation with new reactions on a large scale usually results in
spectacular failure . .
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Again, off topic, but large scale reactions are only needed, IMHO, when you are preparing a reagent for a multistep synthesis. But obviously that
first step needs to be well known and practiced. Performing a multi stage reaction with >100-200mmol sclae for the first time is a rael waste,
especialyl if you have a failure at your fifth steps or so. Even in research chemistry, where there can be alot of waste at times, all reactions are
performed on small scale until yields are good, intermeiates caracterized, and reaction conditions optimized before passing to a preparative scale
(whcih might need optimization itself), etc etc. Doing otherwise is the best way to waste alot of money and time, to arrive with less product at the
end in longer times... Logic.
But I too dispise articles were the reactiosn are performed at 1-5mmol sclae, the authors always report >90% conversion, selectivity etc by GC
analysis, and each time I tried it (except a few rare cases) never worked as mentionned, even at a 20mmol scale.. Pur fame-selling.
I guess everyone sees life differently. But when you work for a firm or a research lab, mmol is the way it goes, even when developing reactions that
will eventually be performed at a industrial scale. Throwing in 200g of reagent and saying "well that didin't work, lets try somethign else with
another 200g" will get you fired in no time (this isn't specially directed to Bahophet, just a general comment).
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|