| Pages:
1
2 |
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
CoF3 is indeed a very useful and powerful fluorinating agent but is likely out of reach of most people doing chemistry as a hobby unless they can
order from Aldrich.
AgF and HgF2 and perhaps to some extent SbF3 and SbF5 are far more likely to be obtainable (allbeit hard) and would be feasible to prepare some of
these from HF etc. In fact they would be cheaper, easier to obtain and easier to handle than CoF3 even for a professional. CoF3 AFAIK requires F2 to
generate it which is probably out of most peoples hands.
Indeed C-C-l3-Br makes no sense but that is not what I wrote.
I merely see that this reaction for halogen exchange in tribromo and tri-iodomethanes to F could perhaps be adapted for exchange to Cl using your
starting material CCl3Br to generate CCl4. And I think, if CCl3Br is easy to obtain compared to CCl4 then why the hell not try a reaction that uses
readily obtainable chemicals like AgF and HgF2?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I gleaned two refs from the Chem Rev monograph that are very interesting. The text says that while the source of nitrogen in the Hantzsch is usuallu
ammonia or ammonium acetate, hexamethylene tetramine and aldehyde-ammonia have occasionally been used. Of the four references, one is an early
Hantzsch paper from Annalen, two are from Ber., and the fourth is not accessible online (Bull.Soc.Chim.Fr.) Well, 3 out of 4 ain't bad. So the Ann.
one I will request in Refs, and the two from Ber. I will pull up in BnF.
It is unclear to me whether or not hexamine is the source of only the NH3 or all or part of the H2C=O as well. I guess we will find out in the full
texts. I will post them here.
Another variation is that ethyl 3-aminocrotonate replaces ethyl acetoacetate and this improves yields. I guess because the N is built in.
[Edited on 19-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
I will move my halogen exchange posts to a new thread anyone interested in fluoromethanes or polyhalogentated methanes especially can look there.
I would have thought that tetrachloromethane would be more useful as a solvent than trichloromethane. I certainly prefer the tetrachloromethane and so
handy with it's azeotropes, I have an ample supply but before I got that I looked into chlorinating trichloromethane.
Sauron are you more interested in trichloromethane than tetrachloromethane? Solvent wise, I expect that bromotrichloromethane itself would be fairly
useable, wouldn't it? I doubt it would interfer in many reactions / crystalisations.
I suppose if the dihydropyridine method reports good yields of trichloromethane then it's worth persuing!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Bromotrichloromethane is available to me, and CCl4 and CHCl3 are not. Period full stop. 99% of my interest in this mixed tetrahalomethane lies in its
being convertible into solvents I need, and I need both of the higher chloromethanes. (DCM is not yet a problem here.)
CBrCl3 costs me, delivered, $500 a Liter. Making it into chloroform will lose some mass, making it into CCl4 will still lose some, but not as much.
Tyros will pop up and say "CHCl3 is easy to make." They know how to make 50 ml or so via haloform. Scaling up the haloform rxn is not very simple
and not very safe. Cooling become an issue. Runaways are common. Phosgene can be released. Making 1000 ml via haloform rxn is simply a lot of work and
I'd sooner throw money and chemistry at the problem, this way.
CCl4 is best made by chlorinatng CS2. CS2 is costly and flammable. The product is a mixture of CCl4 and SCl2 and S2Cl2 difficult to seperate. It can
be done via a trick. The sulfur chlorides are nasty, but can be utilized to make Ac2). The other route is chlorination of MeSCN. Again starting
material is costly. This gets you TCT but I can buy that and do. Continuing the chlorination after filtering off the TCT eventually produces a mixture
of CCl4 and sulfur chlorides just like from CS2.
Doable but again, if I can buy and convert a liter at a time, why not?
My only reaction of CBrCl3 per se (apart from its reduction) is reaction with MeCN and sodium dithionite to produce Cl3CNO, trichloronitrosomethane, a
blue liquid related to chloropicrin. This is a model Novichok binary CW component. Now if if one Cl was exchanged with F, i.e.., CFBrCl2, the
product would be a binary Novichok component proper, very nasty and not something I will ever make. I have reasons you see for NOT wanting F-exchange
reagents around my lab.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Klute:
I am awaiting the seminal 82-pqge paper by Aurthus Hantzsch.
The later paper by him involved acetaldehyde-ammonia adduct to make the 4-Me variant (2,64.6=trimethyl-3,5-dicarboethoxy-1,4-dihydropyridine, a
collidine derivative,. The Griess and Harrow paper using hexamethylenetetramine as source of nitrogen and formaldehyde, produced the correct
structure but the results were a little off analytically so maybe this was not such a great idea.
A 1944 J.Pharm Soc Japan reference suggests formamide can be used in lieu of NH3, but apart from a CA abstract, the full text is likely useless and
in any case in Japanese.
So far the Org Syn prep of 2,6-lutidine has clearest details and so I will go dig through its references in hope that similar clear instructions can
be found for use of a 1:1 adduct of formaldehyde-ammonia, or ammonium acetate, etc. Anything but gaseous NH3.
[Edited on 20-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Not a terribly productive day so far.
The 82 page Hantzzsch paper turned out to concern dihydrocollidine-dicarboxylic acid and derivatives rather than my target. That is, it employed
acetaldehyde-ammonia rather than formaldehyde-ammonia. So the 4-position bears a Me group.
So I dug out Volume 14 Part 1 of the Chemistry of Hetrocyclic Compounds series, Pyridine and Derivatives. This includes a review through 1957 as cited
in the Chem Rev paper posted above. Disappointingly, most of the entries on 2,6-dimethyl-3,5-dicarbethoxy-1,4-dihydropyridine preps were from papers I
already have.
I still have a few stones unturned but it appears the Org Syn prep is probably the best one for this specific target to be found. It is also posted
above. Just a matter of using methyl acetoacetate rather than ethyl.
I looked up ethyl (and methyl) 3-aminocrotonate. Available, not too expensive but 7X more costly than methyl acetoacetate. The latter is $70/2.5 L and
the crotonate is $100/500 g. I'll stick with the acetoacetic ester.
Incidentally the mysterious 20 drops of ethylamine added to the formalin soln and acetoacetic ester in the Org Syn prep, without any explanation or
obvious purpose. Nothing in the notes or discussion or text and the the references were rather mingey.
Apparently this is just a pH adjustment, optimum pH is said (elsewhere) to be 8.5, mildly basic.
Here's a 1902 paper by Knoevenagel and cowoker, prep details are on p 1791.
180 g ethyl acetoacetate
60 g 40% aqueous formaldehyde
180 g 10% NH3 in EtOH
90% yield (>150 g)
[Edited on 21-10-2008 by Sauron]
Attachment: knoevenagel 1902.pdf (819kB)
This file has been downloaded 647 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The way the mechanism of the Hantzsch Dihydropyridine Synthesis is almost always represented is that 1 mol of acetoacetic ester reacts with NH3 to
form ethyl 3-aminocrotonate while formaldehyde reacts with a second mol of acetoacetic ester to form methyleneacetoacetic ester. These two then reach
in a Michael condensation and cyclicization.
That the substitution of ethyl 3-aminocrotonate for one of the equivalents of acetoacetic ester, not only works buts ups yield, supports this
mechanism. However, according to "Pyridines and Derivatives" Part 1, in the Chem.Het.Compounds servies (Vol 14) the mechanism has not been rigirously
proven.
Good old Alan Katritzsky at U Fl did a mechanistic study that may well be more recent than those remarks. I will see if I can get my paws on it.
[Edited on 21-10-2008 by Sauron]
Attachment: ARKmechhantzsch.pdf (580kB)
This file has been downloaded 1064 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
CBrCl3 + Toluene -> Benzyl Bromide + CHCl3 !
We have a whole new ballgame:
It turns out that bromotrichloromethane is a brominating reagent.
Under the action of UV, arenes such as toluene are brominated and the hydrogen abstracted forms chloroform from the trichloromethyl radical. JACS and
JOC call this preparatively useful.
I am chasing the papers.
Here's one of the key papers from JACS attached.
The author worked on a half molar scale in Pyrex using a G.E. 275 W sun lamp for illumination place 6" under the flask and kept the flask cooled with
air at 60 C. Time 6 hours. With various substrates he obtained conversions from 34% (toluene) to 72% (ethylbenzene). In every instance there was a
very close agreement between the molar amounts of chloroform and a-brominated arene recovered. Unconverted CBrCl3 and substrate were also recovered
quantitatively.
Bromination and H-abstraction occured exclusively at benzylic positions. Dibromination was observed only in the pase of p-xylene where a small amount
of p-methylbenzal bromide formed.
The author's photochemical setup was far from optimum. A quartz immersion well and 400-450 W Hg arc medium pressure lamp would be more efficient and
this is the equipment I have operable on a multi-liter basis.
It appears that this can be optimized easily ans scaled up conveniently to 10-20 molar basis (1-2 Kg CBrCl3) - after all the stuff is 2 Kg to the
liter!
[Edited on 22-10-2008 by Sauron]
Attachment: sauran2.pdf (395kB)
This file has been downloaded 933 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If the photolytic bromination of the arene can be driven to completion or nearly so then we are left with only chloroform and benzyl brmide, > 100
C delta in bp's.
Else, we have a mix of the arene, BrCCCl3, chloroform and the a-bromoarene. For the simplest example, toluene, bp's are as follows
chloroform 62 C
toluene 105 C
bromotrichloromethane 111 C
benzyl bromide 198-199 C
Since we do not have to seperate the toluene and CBrCl3, which will still be in equimolar amounts, the workup is easy.
Strip chloroform.
Fractionate the cut boiling between 80 and 115, that is the mixed starting materials to be recycled
Pot residue is benzyl bromide with a trace of hexachloroethane.
Chloroform, remember, is not allowed to be imported where I am. Well, neither is benzyl bromide! Probably because it was long ago used briefly as tear
gas. (See Sartori.) Acros lists it for $300/liter.
I need CHCl3. Getting the benzyl bromide as a byproduct is great and toluene costs almost nothing.
I can exploit the bromide to make phenylacetonitrile (I have a Kg or two already but hey.) I can exploit that to make BOC-On. (Sorry, I do not need
PAA.)
Arenes that a-brominate in this fashion more raspidly than toluene include p-xylene, ethylbenzene, n-propylbenzene, and sec-butyl benzene. All are
costlier than toluene and I have no need whatsoever for the resulting a-bromo compounds. So I will stick with toluene.
This looks like fun.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I am looking at various Hantzsch intermediates.
-- Ethyl 3-aminocrotonate - prepared by bubbling a rapid stream of anhydr NH3 into neat acetoacetic ester for 5 hours w/stirring. In first hour temp
rises to 40 C, and is held to 35-40 with cooling. The product is taken up in ether and water of reaction seperated and discarded. The ether is
stripped off and the product distilled in vacuo. Paper posted below
-- ethyl acetopyruvate - this one has a Org Syn prep. Will post shortly. Prepared from diethyl oxalate and acetone via sodium ethoxide.
-- The Knoevenagel condensation product of formaldehyde and acetoacetic ester, I have not decided as to the best name. Most likely from anhydrous
formaldehyde and acetoacetic ester in a manner analogous to the prep of the enamine above. Possibly from paraformaldehyde or trioxane and acetoacetic
ester. No refs yet.
[Edited on 23-10-2008 by Sauron]
[Edited on 23-10-2008 by Sauron]
Attachment: baminoenester.pdf (426kB)
This file has been downloaded 614 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Nice find! Obtaining equimolar amounts of toluene and BrCCl3 is indeed very practical, and you could make large batch-type reactions, or even
consider a semi-continous operations (distilling the chloroform as it forms, and introducing trecycled toluene/BrCCl3 fractions gradaully). I'm sure
removing the chloroform as it forms with help the reaction proceed, although with higher temps you might get a little more tarry residu..
Concerning the ethylamine: my interpretation is that the ethylamine forms the enaminone more easily than NH3, whcih then condenses with a mole of the
crotonate, forming the cylcisation product and expelling ethylamine, which would be a better leaving group than NH3. I guess the pH issu could also
come into play.
BTW, I have lawys found ethyl and methyl acetoacetate perfectly exchangeable in most reaction, including aminolysis etc (reactions involving departure
of the alcohol moeity)
Looking forward to see how this goes.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks. I think it will be very practical.
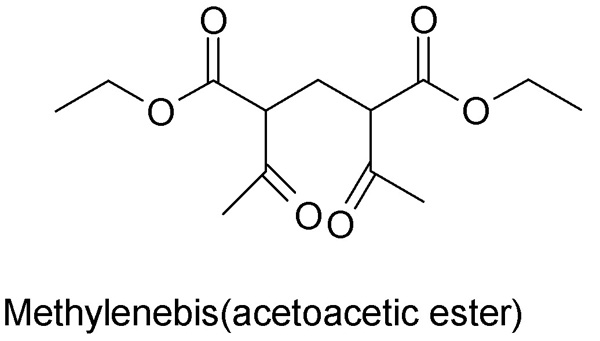
Regarding the Hantzsch, I had a look at the ACS monograph on formaldehyde from forum library. Knoevenagel was first to condense acetoacetic ester and
formaldehyde (as formalin) in 1894 and he used pyridine or diethylamine as catalyst. The product was methylenebis(acetoacetic ester), a 1,5-diketone,
which is immiscible with water and seperates out as lower layer, and can be seperated and dried but not distilled.
Treating the preformed methylenebis(acetoacetic ester) with anhydrous NH3 is what closes the ring.
If you read the Org.Syn. prep of 2,6-lutidine posted above this is exactly what they did in first step.
So the mechanism of the Hantzsch as elucidated by Alan Katritzky (also posted above) and described in your Patai-series book on enamines, certainly
holds for the three component reaction where formaldehyde (or other aldehyde) and acetoacetic ester (or other active methylene) and ammonia (or other
PRIMARY amine) are mixed simultaneously. And in a ratio of 1:2:1.
But if the reaction is conducted as a sequence of two 2-component reactions, for example above in absence of ammonia, there's no enamine formation, or
if an enamine forms from the catalytic (a few mol%) secondaty or tertiary amine, it is solely as intermediate to the methylenebis diketone which is
isolable.
A secondary amine can form an enamine but not an imine, and I am a little fuzzy about whether or not a tertiary amine can form a (quaternary) enamine
at all, I'd guess not.
The authory in Org.Syn waited 48 hrs after condensing the formalin soln and 2 mols of acetoacetic ester, and the hydrophobic lower later formed just
as described in the ACS monograph, and only after isolation and dilution with ether did they introduce dry NH3 and obtain the dihydropyridine.
The methylene bridge forms at the active methylene carbon C3 of each of the two mols of acetoacetic ester. If we draw this you can readily see what
will occur with introduction of NH3 and I do not have to put in a bunch of curved arrows, do I?
We already know (as I spent a couple days digging up the refs) that the enamine can also be preformed (ethyl-3-aminocrotoante) from acetoacetic ester
and dry ammonia, and isolated. It is sold commercially. So one could do that as Step One and then introduce formaldehyde and a second equivalent of
acetoacetic ester, and certainly get same product.
But that is not how McElvain did it in Org.Syn. and that procedure appears to be the gold standard, often referenced by later workers as the paradigm.
[Edited on 24-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Here are the pages from the ACS monograph on Formaldehyde (forum library.)
As you will see, Knoevenagel's original reaction of formalin and acetoacetic ester (2 mols) catalyzed by Et2NH or pyridine, produced cyclic
condensation products (or so he thought.)
Ref.17, Ann. 1894 which I will post soon.
The isolation of the methylenebis(ethyl acetoacetate) was done a decade later by other workers
Ref 24, Ann. 1904, which I will also post shortly.
[Edited on 24-10-2008 by Sauron]
Attachment: Pages from formaldehyde.pdf (861kB)
This file has been downloaded 804 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
And here is the Paul Rabe paper from Ann. in 1904.
The original Knoevenagel prep paper from 1894 is 100+ pages and 3.6 Mb so too large to post here. Anyone interested can find it in References if they
want to read it, it is in a rar file on RS along with this Rabe paper.
For comparison here is the pertinent paragraph from the Org Syn prep of same compound:
"To 500 g. (3.85 moles) of freshly distilled ethyl acetoacetate in a 1-l. flask, set in ice and well cooled, are added 152 g. (2 moles) of 40 per cent
aqueous formaldehyde solution and 20–25 drops of diethylamine. The flask and contents are kept cold for six hours and are then allowed to stand at
room temperature for forty to forty-five hours. At the end of this time two layers are present, a lower oily layer and an upper aqueous layer. The
layers are separated, and the aqueous layer is extracted with 50 cc. of ether. The ether solution is added to the oily layer, and the resulting
solution is dried over 30 g. of calcium chloride. The ether is then removed by distillation on a steam bath. The residue, amounting to approximately
500 g., is diluted with an equal volume of alcohol and is thoroughly cooled in an ice bath. Ammonia is then passed into the mixture until the solution
is saturated. This requires from four to eight hours, and during this time the flask is kept packed in ice. The ammoniacal alcoholic solution is
allowed to stand at room temperature for forty to forty-five hours. Most of the alcohol is now evaporated; the residue is cooled, and the solid
1,4-dihydro-3,5-dicarbethoxy-2,6-dimethylpyridine is removed from the remaining alcohol on a suction filter. The dried ester melts at 175–180° and
weighs 410–435 g. (84–89 per cent of the theoretical amount)."
It will be interesting to compare that to the procedures of Knoevenagel (1894) and Rabe (1904) while noting that the Org.Syn prep was authorored in
the mid 1930s.
And while the rest of that prep is a good read it is entirely concerned with oxidizing the dihydropyridine to pyridine, saponifying the ester groups,
and decarboxylating the compound to obtain lutidine. Not my target. I will be following the procedure in paragraph above, merely substituting methyl
acetoacetate for ethyl, which, Klute reminds us, is a piece of cake.
[Edited on 24-10-2008 by Sauron]
Attachment: PRabeAnn1904.pdf (802kB)
This file has been downloaded 698 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I guess I ought to have a closer look at the Knoevenagel reaction, and reread the Katritzky paper on the mechanism of the Hantzsch dihydropyridine
synthesis. I would like to know whether the 1:1 condensation product of acetoacetic ester and formaldehyde that he spotted by NMR is isolable.
I am pretty sure that the ARK mechanism applies only to the formal, 3-component Hantzsch. In fact it is pretty clear that procedures like the one in
Org Syn are not precisely Hantzsch reactions at all, although they involve the same reagents and arrive at the same products, simply because they
aren't 3-component reactions. I don't think this is splitting hairs.
I did have another go at ARK's mechanism paper and he does include the methylenebis-ethylacetoacetate in his master reaction scheme and obviously
recognizes the complexities. Well, I really would not expect any less from Katritzky.
His experiments were using benzaldehyde not formaldehyde.
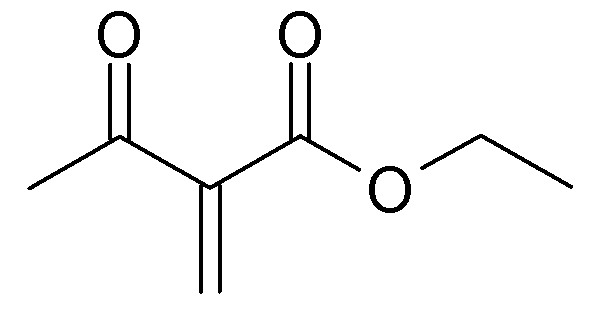
Below is theethyl 2-methyleneacetoacetate resulting from condensation of one equivalent acetoacetic ester and one equiv. formaldehyde. Just as
clearly, we could call this ethyl 2-acetylacrylate.and expect it to be highly reactive and readily polymerizing. I am a little dubious about whether
or not this can be isolated, except maybe in a cold trap. where the self-condensation can be retarded. But ARK's spectral evidence for in situ
formation and role in reaction with ethyl 3-aminocrotonate is pretty compelling.
The ACS Formaldehyde monograph described methylenemalonic esters and methylenebis(malonic esters) and as anticipated these do polymerize on standing
but not instantly, and are isolable and distillable. However, no mention is made of a methyleneacetoacetic ester, just the methylenebis compound. I
will have to dig deeper.
[Edited on 24-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
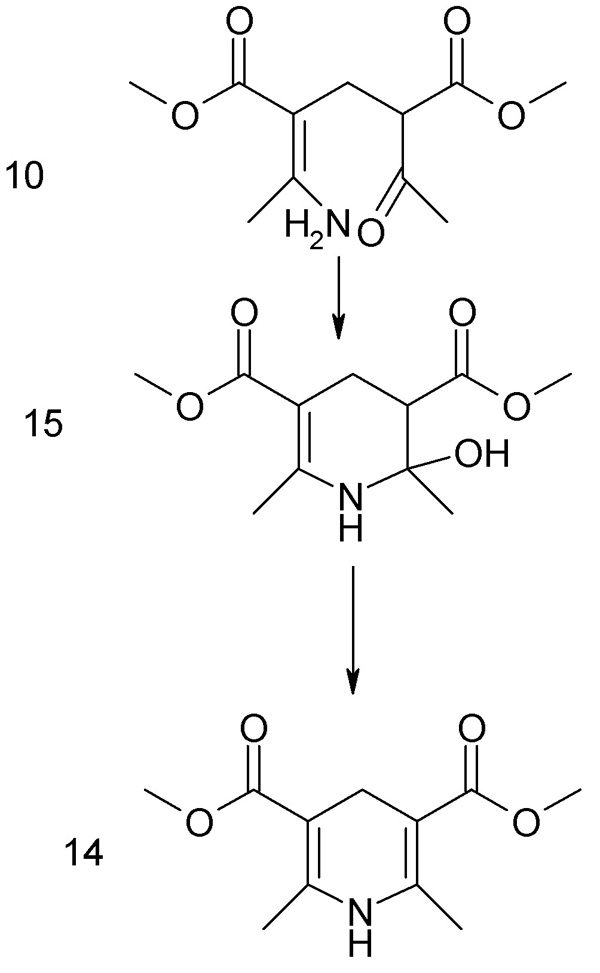
The Katritzky paper posted above concludes from NMR experimental evidence that the main pathway of the three-component Hantzsch is (by reference to
his Sceme 1 diagram) enamine 5 + Knoevenagel product 6 -> Michael addn product 10 -> ring closure product 15 ->dehydration product 14.
The enamine (in case of our target, this is methyl 3-aminocrotonate) forms within minutes of addition of ammonia, while clalcone 6 takes a few hours.
The dienamine and the 1,5-diketone were not observed. - that is, were not detectable by NMR under these experimental conditions.
Did Katritzsky load the dice by using benzaldehyde rather than formaldehyde? I wish I had a NMR so I could find out. In the meantime I guess his
mechanism is the best game in town.
[Edited on 25-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
In my sketch above, 10 is the Michael addition product of enamine 5 (methyl 3-aminocrotonate, formed from ethylacetoacetate and NH3) and 6 the
Knoevenagel chalcone formed from a second equivalent of methyl acetoacetic ester and formaldehyde. The enamine forms faster than the chalcone.
15 is the ring closure product with loss of H2) from 10
14 is the final dihydropyridine from loss of H2O forming the second pi bond.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Eureka!
I have found what appears to be the solution of this problem.
According to the attached US patent to Dow Chemical, antimony, tin, zinc or vanadium chloride catalyzes the elemental chlorination of alkanes with at
least one substitutable bromine.
In their first example, Br2CCl2 is chlorinated to BrCCl3 using Cl2 and SbCl3. No photolysis, no pressure, just reflux.
So CCl4 can be prepared this way.
Most likely the chlorine adds to SbCl3 in situ forming SbCl3, the released bromine also adds to the antimony catalyst forming SbCl3Br2 or SbCl2Br3, so
the catalyst accelerates the chlorination and retards bromination reverse reaction. This is similat to antimony chlorofluorides in fluorine exchange
reactions. The patent makes it clear that SbCl3 is the preferred catalyst along with SbCl5 and mixtures of the two.
[Edited on 19-2-2009 by Sauron]
Attachment: US4197262.pdf (154kB)
This file has been downloaded 555 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
| Pages:
1
2 |