stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
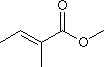
Oxidation of Methyl Tiglate
When oxidizing the double bond (to an epoxide or diol with a peracid or H2O2), is it likely that the carboxymethyl group will come off?
[Edited on 17-7-2009 by stoichiometric_steve]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Being an electon deficient alkene your best bet would be to use H2O2/NaOH. Following the mechanism I would expect the epoxidation to occur fine, as
the other product would be a ketene. However you are still plagued by the basic conditions under which the methyl ester could be hydrolysed. How do
you suspect the carboxymethyl group to come off? Do you have any mechanistic reasoning to assume so?
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
When using a strong peracid, the resulting epoxide ring could be opened, and the ester could also be hydrolyzed which would leave the carboxyl group
prone to coming off. Actually, i didnt really think about the whole thing thoroughly, since it's quite late here already. But thanks for your input,
it is greatly appreciated 
Edit: Using NaOMe with H2O2 would certainly reduce the probability of ester hydrolysis.
[Edited on 17-7-2009 by stoichiometric_steve]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I would try a buffered biphasic peracid oxidation: the product will be protected from too acidic conditions, and using a PTC would propel the peracid
in the organic layer.
What's your plan with this compound? Is this part of a multistep synthesis?
[Edited on 17-7-2009 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Here is a tetrahedron review of epoxidation mechanism.
Methyl Methacrylate epoxidation is covered in the review so hopefully it will provide some insight. 
Decarboxylation may be a problem as joe90 said, but I think with low temperature and good choice of mild reagent it can be avoided. If starting with
carboxylic acid then decarboxylation to ketone probably bigger problem.
Attachment: Mechanism of Olefin Epoxidation.pdf (991kB)
This file has been downloaded 966 times
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
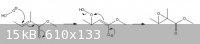
I know the mechanism for epoxidation of olefins with peracids - the problem is that this alkene is electron deficient (due to the carboxylic acid
group - its a,b-conjugated) and so should react best with basic H2O2. Steve's suggestion of using methoxide is a good one - with a large excess of
methoxide the reaction should work nicely, but as hydroxide is a product of the epoxidation then it begins to hydrolyse the ester - keeping the
methoxide concentration high will minimise this (I'm thinking >5 eq.?). I'll attach the mechanism for this reaction:
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
DJF90, do you have any refs for basic H2O2 oxidations handy?
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I see no reason not to use m-chloro-perbenzoic acid here. The ester is not easily cleaved in such mild acidic condtions, though it will surely cleave
even in weak base. The mechanism is "concerted", so it's not really appropriate to show nucleophilic addition to the alkene, which is here a Michael
acceptor. MCPBA it.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Except for its prohibitive price tag. I would like to stick to peroxide or a low M.W. peracid, if possible.
|
|
|
Formula409
Hazard to Others
  
Posts: 129
Registered: 13-12-2008
Member Is Offline
Mood: No Mood
|
|
Payne, Deming, & Williams, J. Org. Chem. Vol 26, p569 (1961)
I've also requested it in the refs forum for you, will post when it has been retrieved.
Formula409.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Formula, thx. I knew about this one, and it is also described in Org.Syn.
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv7p0126
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I'm sure I have a couple, I'll check my books in a sec. Arrhenius - I think you will find that m-CPBA will not work here, the substrate is an
a,b-unsaturated ester meaning that the "alkene portion" is electrophilic rather than nuclephilic - as such it will not attack the m-CPBA (thats the
first step of the concerted reaction - the alkene "reaches out" and attacks the oxygen). I have already mention this upthread.
The references I have are:
Weitz, E.; Scheffer, A. CB 1921, 54, 2327
Corey, E. J.; Ensley, H. E. JOC 1973, 38, 3187
Itsuno, S.; Sakakura, M.; Ito, K. JOC 1990, 55, 6047
I trust you are capable of getting these yourself - they seem to be for a,b-unsaturated acids and ketones, but esters should react the same as
departure of the alkoxy group would yield a ketene (follow the mechaism and you'll see what I mean).
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Here is better paper than mechanism crap I posted before! 
Peracetic acid as oxidant and decent yields 
Attachment: Epoxidation of a,b-Unsaturated Esters with Peracetic Acid.pdf (567kB)
This file has been downloaded 1214 times
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
DJF90 - Yes, I know you already mentioned this, and I disagreed. I know it's a Michael acceptor, but that doesn't mean it can't be a nucleophile.
But anyhow... I think there's absolutely no question that your hydroxide ion is going to 'reach out' and saponify the ester... I've run saponification
with 1eq of base stirring overnight; sounds like the epoxidation reaction requirements.
I think MCPBA will work. MCPBA is cheap, so i'll assume what 'too expensive' means is 'unavailable' or 'i'm making this on multi-kg scale for
profit'. peracetic or performic would be my second choice. Don't bother with the buffering, just run on a small scale and see what you get.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
I'm essentially trying to keep the whole process as "green" as possible.
Besides that, roughly 400 EUR per kg of mCPBA isn't exactly what i would call cheap.
[Edited on 18-7-2009 by stoichiometric_steve]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Arrhenius - I see your point. However a nucleophilic HO-O(-) species is likely to react with the michael acceptor at a much higher rate - the long
reaction times should not be needed, and if NaOMe is used as the base then hydrolysis should not be that much of a problem. Albeit still a problem.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Can't use methoxide in water as it yields methanol and hydroxide. Can't use peroxide without water as it explodes. The rate of ester hydrolysis is
quite fast.... I don't know, I just don't see it going well.
The rate of ester hydrolysis under acidic conditions is much slower than in basic conditions, and the water is less of an issue.
MCPBA does not contain water, but it's also a good portion benzoic acid (pure MCPBA explodes). Peracetic acid and performic acid cannot be prepared
without water as anhydrous hydrogen peroxide is unavailable.
[Edited on 19-7-2009 by Arrhenius]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Good points. I know of a compound that can be used to yield anhydrous peroxide in situ - Steve can PM me for details. I hadnt considered the presence
of water beforehand though - thanks for drawing my attention to that. Ester hydrolysis will happen regardless, as m-CBA (a byproduct of m-CPBA
oxidation) will act as an acid catalyst. Assuming the rate of basic hydrolysis is approximately equal to acid hydrolysis (a big assumption I know)
then surely the reagent that epoxidises fastest is the one that is best to use for this specific reaction.
Ok you edited before I posted so now some of what I said is obselete.
[Edited on 19-7-2009 by DJF90]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
You can use sodium perborate to generate peracids in-situ with minimum amount of water. Yje only water introduced would be the water from hydratation
of the perborate, and the generation of the peracid.
I think perborate can be used in basic conditions too for epoxidation.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Yes it should work well. And no doubt the perborate and methoxide required will be cheaper than the m-CPBA, and also greener, which was one of steve's
points upthread IIRC.
|
|
|