Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
5-Isopropylresorcinol Questions
Im am attempting a synthesis of an organic lignand which has 4,6 Benzhydrl-5-amino-cumene as its main constituent. My plan to synthesize this compound
is by brominating 5-isopropyl-resorcinol to 4,6 bromocumene and then nitrating it to 4,6-bromo-5-nitro-cumene. Then react that with Mg to form a
gringard reagent, and use a Kumada coupling reaction between it and bromodiphenylmethane to form 4,6-benzhydrl-5-nitro-cumene. Then the nitro group
would be reduced to a amine using PtO2.
Does this synth seem like it would work? I also am only going to try it if I can find a way to make 5-isopropylresorcinol or if my inquiry about its
price is something I can afford.
Is there anything you guys can find on a synth for 5-isopropylresorcinol? I cant really find anything. Could I use a Friedel-Crafts Alkylation with
resorcinol's methyl ester and isopropyl chloride to form it?
Thanks,
Jackson
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
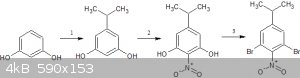
1https://pubs.rsc.org/en/content/articlelanding/1910/ct/ct910... (use isopropyl alcohol instead of methanol)
2https://www.tandfonline.com/doi/full/10.1080/15533174.2011.5...
3.appel reaction
after this you can do kumada coupling followed by reduction to get the target compound
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Okay, thanks so much.
So the nitration will favor the 5 position?
I assume so but I just want to make sure.
Thanks,
Jackson
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
CuReUS
For the first reaction, I cannot find how that paper has any mention of creating a methyl resorcinol from resorcinol. I must be missing something,
could you tell me what you are refering too?
Thanks,
Jackson
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Jackson  | CuReUS
For the first reaction, I cannot find how that paper has any mention of creating a methyl resorcinol from resorcinol. Jackson |
Sorry I made a mistake.The paper uses m-cresol,not resorcinol.I mistook the methyl group of m-cresol for OH  .This means we can't make 5-isopropyl resorcinol that way .This means we can't make 5-isopropyl resorcinol that way
the another thing you could do is to make 3,5-dihydroxybenzoic acid(from resorcinol or benzoic acid),do a kochi reaction to get 5-bromoresorcinol and
then do a suzuki coupling with 2-bromopropane to get 5-isopropylresorcinol
[Edited on 26-1-2019 by CuReUS]
|
|
|