avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
Practical home synthesis for Formaldehyde
i really need to get formaldehyde, to obtain hexamine for my rdx synthesis, so i read about methanol dehydrogenation over heated copper which form
formaldehyde "ch3oh -> ch2o + h2" so i decide to build a little reactor which evaporate methanol fumes to a copper pipe which rust inside with his
oxide catalyst and heat it to 300c, and i don't know the efficiency of this process.... i heard about it somewhere that the formaldehyde is over react
with the copper catalyst to form formic acid and co gas....
someone know the efficiency of methanol dehydrogenation over heated copper?.... or know effective way to get formaldehyde or hexamine?(i don't have
access to fuel tablets)
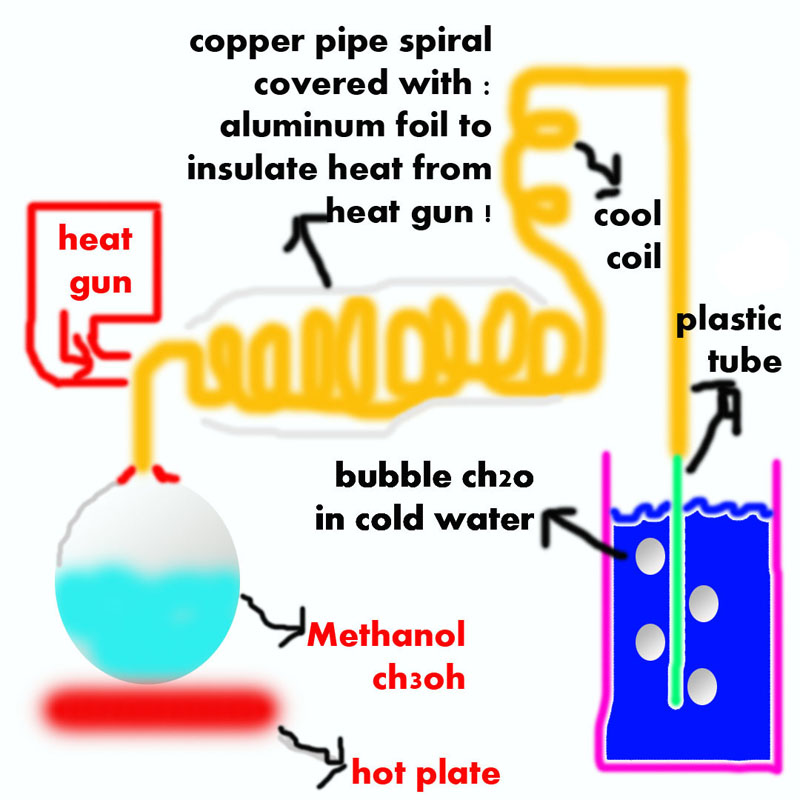
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | (i don't have access to fuel tablets) |
I've seen hexamine fuel tablets on eBay and other places!
My first hexamine was from formalin and ammonia cleaner - later from a camping-supplies shop!
I get formalin by the gallon from my local pharmacist. . .
Preparation of formalin looks like a real pain when a bit of seaching might suffice!
|
|
|
gnitseretni
Hazard to Others
  
Posts: 282
Registered: 5-1-2007
Location: Colombia
Member Is Offline
Mood: No Mood
|
|
What about heating paraformaldehyde?
|
|
|
mickelcorleone
Harmless

Posts: 2
Registered: 5-4-2010
Member Is Offline
Mood: No Mood
|
|
I think that the best way for formaldehyde synthesis
you can use PCC OR PDC
YOU CAND DIRECT SYNTHESIS BY PCC
CH3OH_____>ADD PCC----->H2C=O

|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
pcc i carcinogenic and more hard to get then formaldehyde, by the way its made from formaldehyde.
somebody can tell me what he think about the process which i mentioned above please?
if someone know practical way to synthesis this stuff please post it.
[Edited on 8-4-2010 by avi66]
|
|
|
Nicodem
|
Thread Moved
8-4-2010 at 13:02 |
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by avi66  | | so i decide to build a little reactor which evaporate methanol fumes to a copper pipe which rust inside with his oxide catalyst and heat it to 300c
|
A couple of comments purely on the engineering.
Assume inefficiency, which means you have plenty of unreacted methanol in your output vapor. The sketch of your apparatus doesn't have a way of
separating these two vapors. You should be able to put a temperature controlled condenser in the line, after the catalyst bed and before the water
trap, and recycle back the bulk of the methanol back to the boiler. This way, the consequence of catalyst inefficiency is a slower output rate, rather
than a second-stage separation procedure. You might not care about this separation step, though.
Also, you'll want more surface area than the inside of a circular pipe, which is a minimal surface. Pack the heated catalyst pipe with copper mesh.
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
ן Have lcd hot air gun with temperature control, and about the methanol recycle, its not my problem,i can make condenser and recycle the
methanol.
i dont afraid from low efficiency .... i afraid of spending the methanol ... i don't want it to became formic acid and other compounds .... i want
most of the methanol 90%+ which will react, will form ch2o.
so here i ask you guys if you know the ratios of compound which form in those conditions.... and about the surface area .... its very long and dense
spiral, and i already flat the pipe, so i got a lot of surface area.
Thanks for the helpers 
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by avi66  | | and about the surface area .... its very long and dense spiral, and i already flat the pipe, so i got a lot of surface area. |
Less surface area than you think, I'm afraid. Flattening the pipe changes the surface area very little, at most a few percent. It's
quite instructive to take apart some copper mesh (it's usually knit and can be undone from one end) and wind it around a cylinder form just to see how
much area there is.
Now, as far as your yield question goes, if you don't have any references from when you read about this in the first place, perhaps you might be
asking how to find such references rather than asking others to be your librarian.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
OTOH, you could (pretend to?) take up taxidermy and legitimately look for formalin in your area.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
New members who are too lazy to search
Must we have yet another thread on preparing HCHO by oxidation of CH3OH?
There are literally dozens of threads on formaldehyde already, including this one which contains lots of useful information, including references.
Must we clutter up the forum with the latest kid's sad attempt at chemical engineering?
IMHO anyone without the imagination to figure out how to obtain formaldehyde probably (certainly?) won't be able to make it from methanol.
It's one thing to discuss novel synthetic methods that may or may not work. It would be even better if those proposing such methods could present
some experimental evidence that these novel methods might work. But it's just a waste of time to entertain the brain farts of everyone who wants to
know how to make something that's been discussed ad nauseum, just because they're too lazy to search the forum.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Ahh entropy calm down, yes it is annoying and useless to ask to be spoonfed but keep in mind this guy only has 3 posts and obviously not aware of it.
Best thing to do is to nicely remind people of what they are doing wrong. Afterall it is very discouraging for new members to join when people are
being so nasty, infact I didnt join until two almost 2 1/2 years after I found this sight since I was worried members would be overly critical.
Anyway back OT
Avi there are a couple of things to keep in mind if you try this reaction. First and perhaps most importantly methanol vapor is very flammable and if
any sizeable amount of air gets into your setup then you basically have a small FAE. Another thing if you do succeed with carring out this reaction,
you will most likely have a large amount of methanol in your formaldehyde solution wich would need to be separated. Oh you shouldnt get any formic
acid in your product unless air gets in there somehow, since there is only one oxygen in methanol and formic acid has COOH. And if you dont want to do
any of this than I may be willing to send you a link of a supplier who sells 37% formaldehyde for 18.00 a gal, but beggers cant be choosers so I will
see.
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
i take my idea from this site : http://www.ucc.ie/academic/chem/dolchem/html/comp/methanol.h...
that is all i can find , and i guess that if this process was good, its was published on google more then once.
so after long period of time 6 month that im search in google many many times ... i decide to ask professionals.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
I just searched for "dehydrogenation methanol formaldehyde" on Google. There are a lot of references. While most of them are in
academic literature, some are patents and some are available to read, and by "some" I mean "enough to get started" and "enough to have better
questions".
The most striking thing is that there are a large number of catalysts available for this reaction. A lot of what you're asking is irrespective of the
catalyst.
Lastly, I notice that you need a way of disposing of the hydrogen you'd be generating. It's not in your diagram, and should not just be assumed to go
away safely.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Yes.
And "yes", because we want to make more chemists. It's clear that OP has little experience with the engineering issues and just as little experience
researching the literature. On the other hand, OP has enthusiasm, which we should not squander.
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
i do not have taxidermy shop in my area, and the medical formaldehyde have very tiny amount of formaldehyde ... about 4% .... how can i hell get this
stuff ..... i need to explode something dam it !
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I honestly fear for your fingers......
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
I'm afraid my inclination to assist
drops precipitously with this comment, as the presence of explosives in the absence of patience is just dangerous. If you can't be bothered to spend
the requisite time learning the theory, building safe equipment, and methodically performing the procedures, I can't be bothered to help you injure
yourself. I don't particularly care that you want to make explosions, but I do care if you want to do so heedlessly.
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
i am professional with high explosives  don't Worry, i got all the safety
equipment i need, so if someone know a practical way to synthesis this stuff at home, or know the ratio of ch3oh->ch2o +h2 this reaction/(global
methanol conversion), please post it, because i didn't find a information about the, methanol dehydrogenation over heated copper at 300c. don't Worry, i got all the safety
equipment i need, so if someone know a practical way to synthesis this stuff at home, or know the ratio of ch3oh->ch2o +h2 this reaction/(global
methanol conversion), please post it, because i didn't find a information about the, methanol dehydrogenation over heated copper at 300c.
Thanks and good day.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Check this out. . .
http://cgi.ebay.co.uk/BUY-in-BULK-60-x-Mini-HEXAMINE-Fuel-Ta...
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
nice one .... but when its arrived to Israel, the airport will see that i purchased hexamine, they will send the army to my home, because they will
think i am a dangerous terrorist, who want to make cyclonite,  joking .... i am
combat engineering soldier in Israel .... but the airport still band this material. joking .... i am
combat engineering soldier in Israel .... but the airport still band this material.
nobody heard about someone who succeed, with this formaldehyde synthesis?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Sheesh! Now the 'Avi' registers - but doesn't the IDF provide the excitement you seem to crave?
They certainly seem to have little hesitation in expending their munitions for one reason or another. . .
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by avi66  | i am professional with high explosives  don't Worry, i got all the safety
equipment i need, so if someone know a practical way to synthesis this stuff at home, or know the ratio of ch3oh->ch2o +h2 this reaction/(global
methanol conversion), please post it, because i didn't find a information about the, methanol dehydrogenation over heated copper at 300c. don't Worry, i got all the safety
equipment i need, so if someone know a practical way to synthesis this stuff at home, or know the ratio of ch3oh->ch2o +h2 this reaction/(global
methanol conversion), please post it, because i didn't find a information about the, methanol dehydrogenation over heated copper at 300c.
Thanks and good day. |
BS, you are no professional and I personaly think like the airports that you are a terrorist. And thats saying alot from someone whos from a country
that tosses that word around so much it makes my stomach churn.
"i am combat engineering soldier in Israel "
This makes it quite clear your intended use of the formed explosive and I think everyone here should drop you from there mind unless they want the
thoughts of other peoples body parts floating thru there dreams knowing theres responsible for helping the man make the stuff that blewup himself or
countless others. I say ask yor superiors "soldier" and see what they can do for you.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
don't speak rubbish ... pyrotechnic is only a hobbie for me ...  and yes
because this hobbie i join combat engineering unit in Israel army, its stink there i just guard alot. and yes
because this hobbie i join combat engineering unit in Israel army, its stink there i just guard alot.
im trying to make formaldehyde by myself, because there are alot of stupid arabs terrorists in my country which cause a restriction of those chemicals
import, and be sure that arabs which want to use the explosives to bad purpose, will not use a forum ... they are know everything from the terror
organization, i can tell you that as a soldier who know the ability of the bad guys.
i need high explosive in my fireworks, because i really want to create a mini rockets 1.5 cm inner diameter,which carry 20g of rdx high explosive
warheads .... its can be cool, i don't want to hurt people ... Israel army is the most moral army in the world(just to remind you), not all the the
peoples here are bad .... most of the arabs are bad, but the jews in israel are very moral ... like all the advanced country's in the world.
and do not ban me from this forum please ...  i like this forum, there is a
high level of knowledge here. i like this forum, there is a
high level of knowledge here.
[Edited on 10-4-2010 by avi66]
|
|
|