andre178
Hazard to Self
 
Posts: 61
Registered: 11-12-2009
Location: San Diego, CA
Member Is Offline
Mood: pacified
|
|
Developing new reactions
This definitely a newbe question, I have finished going through years of theoretical OCHEM, but have subpar lab experimentation (other than
academically assigned and also a few dabs here and there).
I wish to become proficient in devising my own experiments. We as a science community understand chemistry a lot better than they did 50, 60, 70 years
ago when many of the first chemical synthesis books were first published. Let's not forget that books in the last decades have a hybrid of old methods
intercalated with a few newer ones.
Without denial, the key to becoming a good chemist is practice; the practicality aspect of my experience is not overlooked, but the keys to success is
to have the right tools available, which brings me to my point.
When devising an experiment that may or may not be in an Organic Synthesis "Bible", for example, the following 2 reactions
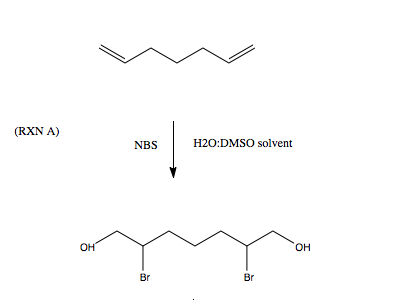
Here were my steps in thinking:
1) I knew I wanted markovnikov addition for alcohols and bromine. So Br2 in H2O should give in theory the chosen product. However Br2 being so
unhindered and reactive would give more side products (ref 1) so NBS is better suitable, which reaction A is based on (ref 2).
2) the equivalents of NBS and alkadiene should(?) be 2:1, basic stoichometry
3) DMSO/H2O, because I have DMSO available, though I am thinking about THF since it has a lower bp (thinking about product purification).
4) the solvent chosen is water (which in this reaction acts as a nucleophile and as a solvent for the product, and the alkene solubility is arranged
by the THF/DMSO/DMS etc.) - even when reading about solvents and reagents (ref 3), I do not fully comprehend which optimal solvent to be chosen.
5) purification of diol, unless the product is a solid, seems I doubt, may be a little complicated: getting rid of NBS and DMSO/THF (which are good
solvents). Hypothetically if the next step is protection of alcohols, could I just add pyridine and Ac2O, or this is considered bad practice and
doubtful results?
side question: how is the input heat of the reaction determined? trial and error or a computational way of doing it?
Thanks for reading, it was a lot, but there's so many questions that as a newbe, I've come across that no book really answered, and often professors
just give textbook type of answers.
References
1. Wiki: Halohydrin formation rxn
2.A guide to solvents and reagents
3. NBS
[Edited on 5-10-2010 by andre178]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Isn't the Br going to end up on the terminal carbons?
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
There are reported HOBr additions in DMSO/H2O. However, I also don't see this reaction working effectively. NBS forms hypobromite, which reacts with
alkenes in a Markovnikov fashion, so you'll get a bis-bromohydrin with bromine at the terminal positions. You'd need to use an excess of NBS.
I don't want to be a downer, but chemistry is a very well developed field at this point. Developing new reactions is truly an immense challenge. The
chemistry 'bible' is the primary literature, and this is ridiculously large collection of work. Don't fool yourself, chemists even 100 years ago were
quite knowledgeable - you would be surprised. The likelihood of you being able to develop a 'new' reaction at home is slim to none. Professional
researchers devote an unbelievable amount of thought, time and effort to developing even a single new methodology or reaction. You're better
off buying more advanced textbooks (March's Organic Chemistry, Zweifel & Nantz - Modern Organic Synthesis) and building on your knowledge from
there. As for what you'll be able to achieve at home, this is dependent on how much you're willing to break away from the 'otc' mantra. You
really need to convince yourself that it's okay to purchase intermediates and reactants from chemical suppliers if you want to do some clever
chemistry. I've got plenty of legal ideas, so surely you do too 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Andre178, what you are asking is about designing a new synthesis, not developing a new reaction. The reaction you ask about is way over a century old.
It is called electrophilic addition on alkenes, more specifically it is about bromohydroxylation.
What you need to do is to determine the most likely products first. For this you use mechanistics and primary literature. Thus, you need to check the
mechanism of bromohydroxylation (electrophilic addition on alkenes) and search the literature for this specific or related synthesis/method/product.
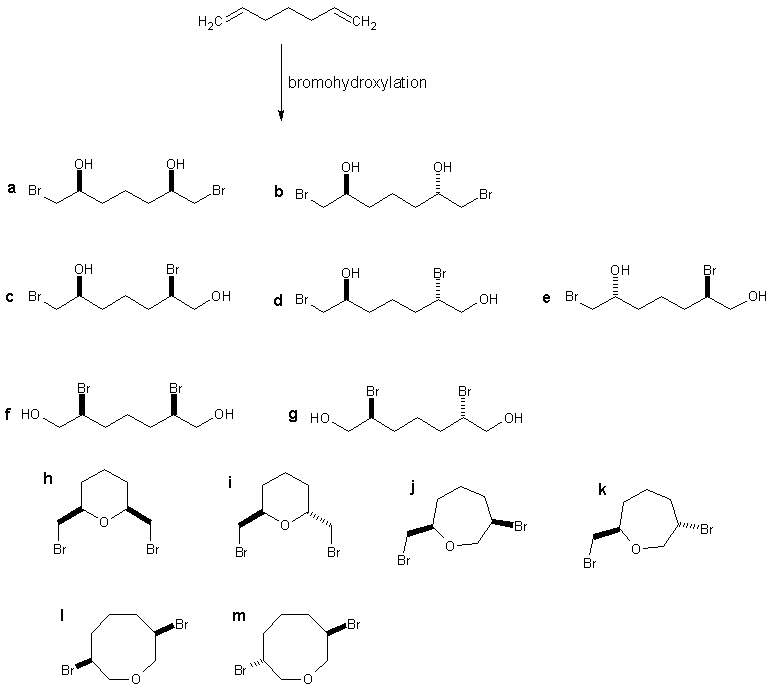
For example, it helps if you first make a chart of all possible bromohydroxylation products of your substrate and then rule out the unlikely ones
(those not conforming the regioselectivity, stereoselectivity, statistics, ring size, Baldwin's rules and so on). Bellow is an example of few such
products, but they do not include the non-bromohydroxylation products, the ones breaking the (anti) stereoselectivity of the nucleophilic attack on
the bromonium ion and few other things (it would be too much to include everything, just to rule out immediately). They do however include products
that are unlikely due to incorrect regioselectivity (c, d, e, f, g) and/or products that are unlikely due ring size thermodinamics
(j, k, l, m). Regioselectivety of reactions is often non-specific, that being, some reactions commonly give mixtures of regioisomers,
so products derived from lack of regioselectivity should not be immediately ruled out without consideration.
You see that just applying a few basic rules you rule out a whole bunch of products. Applying statistics gives you the information that the amount of
a formed will be more or less equal to b, as the diastereoselectivity is expected to be low, due to the lack of
steric influences. Same goes for h and i diastereomers. Applying the knowledge of thermodinamics and Baldwin rules
will tell you that an intramolecular reaction is more likely than a intermolecular one, thus giving you the idea that the products h
and i should form in higher amounts than a and b, but this is obviously highly dependent on the
concentration of H2O and method used. Furthermore, the awareness that you are dealing with a symmetric reaction will give you the information that all
the products formed will be racemic.
This is only about the reaction and you also need to consider the method. If the use of NBS in aqueous DMSO is not yet described for simple terminal
alkenes like the one in question, you need to think about the possible side reactions. Haloimides react with DMSO (with the rate highly depending on
the haloimide used, the pH, temperature and other parameters), so if the desired reaction is slow, some NBS will be consumed by this side reaction.
Carbocations get oxidized by DMSO to give ketones/aldehydes. Since bromonium ions are in equilibrium with the appropriate carbocation, you can only
hope the nucleophilic addition of H2O on the bromonium ion is faster than the nucleophilic addition of DMSO, else this becomes another possible source
of side products. Side reactions consuming/reducing NBS give bromide as one of the side products and this can interfere with the bromohydroxylation.
It can react with NBS to give Br2 which can add to the alkene or it can nucleophilicaly add on the bromonium intermediate, in both cases leading to
1,2-dibromoalkanes instead of bromohydrines. So the choice of bromohydroxylation method is important if you want to reduce the amount of
1,2-dibromoalkanes side products.
This were just examples of a few things to consider and there are more!
If you ever graduated from organic chemistry you should have been thought all this already (if not, make a reclamation of your schooling!). What you
need when planing a synthesis is being able of analytical and divergent thinking, and being able to perform primary literature searches. The first is
highly dependent of the culture you were raised in, the schooling system and your ideological background. Unfortunately, in the last few decades most
educational systems in the world are supporting convergent thinking and suppressing divergent thinking, so schooling can often be detrimental for
those pupils who would want to become researchers. The ability of performing primary literature searches depends on you learnt skills as well as
access to abstracts databases and literature, but unfortunately it is also highly dependent on your personality.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Ouch.. I'm going to disagree on any formation of 7 and 8 membered ring ethers by Sn2. May be 'allowed' by Baldwin rules, but highly unlikely to form.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | | Ouch.. I'm going to disagree on any formation of 7 and 8 membered ring ethers by Sn2. May be 'allowed' by Baldwin rules, but highly unlikely to form.
|
I agree that rings other than the 3, 5 and 6-membered are always harder to form and this is one of the reasons I ruled out those 7 and 8-membered
j, k, l and m products already in the first stage of analysis. But this was not the only and main reason. The other
important reason is in that these can only form if the first bromohydroxylation occurs against the proper regioselectivity.
Otherwise, the cyclisation of omega-hydroxy-bromonium ion is not really a classical SN2 substitution. It is not a true SN1 either, because bromonium
ions are not true carbocations, but instead are highly reactive cyclic onium electrophiles which only formally resemble carbocations hyperconjugated
with the neighboring Br. They are extremely reactive not only due to the strong electronic effects, but also due to the ring strain. The consequence
is in that they have their own peculiar chemistry. That the addition of nucleophiles on halonium ions does not go through true carbocations is
especially apparent in the poorer regioselectivity of chlorohydroxylation of simple terminal alkenes (besides, if the reaction involved carbocations
the reaction would be completely regiospecific because it could only go through the secondary alkyl carbocations). On the other hand, if the attack of
the nucleophile on the halonium ions would follow an SN2 mechanism, the bromohydroxylation of terminal alkenes would give 2-bromo-1-hydroxyalkanes
mostly (similarly to the regioselectivity in the SN2 alkylations with non-protonated epoxides or aziridines) rather than the observed
1-bromo-2-hydroxyalkanes (here the similarity of halonium ions and protonated or acid coordinated epoxides and aziridines becomes obvious – all
these are onium ions!).
Also, you would be surprised to find out that the halonium based cyclisations (as for example the above discussed intramolecular bromoalkoxylation or
the related halolactonisations) easily give 7, 8 or more membered rings under the appropriate conditions and reagents used. This is one of the few
reactions that can be used in the synthesis of macrocycles without resorting to dilution. For few examples, see: J. Org. Chem., 66 (2001),
4304–4310 (DOI: 10.1021/jo0017234) and European Journal of Organic Chemistry, 3 (2003) 463-471 (DOI: 10.1002/ejoc.200390080).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
andre178
Hazard to Self
 
Posts: 61
Registered: 11-12-2009
Location: San Diego, CA
Member Is Offline
Mood: pacified
|
|
first off, thank you for the incredibly useful replies, in a sense they are disheartening that there really isn't an easy way to break into chemistry,
especially for a newbe that had many experiments go wrong. The science I am attempting to do is in a lab, but after work, I work in Biology 9-5. I
have permission and access to a lot of chemicals that I request - like a hobby chemist.
Chemistry is not like cooking, nor like a construction of legos, but I'll try to get the hang of it as time goes on. I've been reading March's and
trying to go from there too.
finally, I am glad this forum exists, and thanks again to all folks who take the time to discuss and reply.
Andre
|
|
|
|