rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Seperating Ethanol and 3-methyl-3-pentanol
Apparently Ethanol and my product 3-methyl-3-pentanol are not miscible so a large portion of my product distilled over with the ethanol produced
during the grignard giving me a nice cloudy emulsion. Any ideas on how to remove the ethanol from the emulsion and seperate my product out?
EDIT: It seems to slowly be forming two layers. I'm gonna wait to see if it completely seperates. Then it is the fun of trying to figure out which
layer is my product and which is not.
[Edited on 5-14-10 by rrkss]
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
3-methyl-3-pentanol is supposed to be miscible with ethanol and soluble in water at 45g/L.
Maybe adding concentrated salt solution would dissolve the ethanol but salt out the product. CaCl2 is also supposed to absorb alcohols.
Congrats on the home-made Grignard.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
I've seperated the two layers. The bottom layer has a very acrid almost burning like smell. The other layer smells like pine trees to me. The
reason I guessed it was ethanol was because it came over at 77 degrees. But maybe my product forms some sort of azeotrope or is just following
Rault's law for immiscible liquids.
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 857
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Why don't you distill it? The difference in boiling points are about 45*C. A short column or an anti-splash adapter should improve the separation.
[Edited on 14-5-2010 by Lambda-Eyde]
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Don't own a short column. Would a 70 mm straight adapter be a good substitute?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Rrkss, have you forgotten to dry the extract over Na2SO4 before distilling?
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
I used MgSO4 to do the drying. The emulsion cleared up and I have two distinct layers. I am wondering if the second layer is the alkyl bromide of my
product giving that it is a tertiary alcohol and I did go a bit overboard acidifying my grignard after completion. Maybe HBR formed insitu from the
MgBr(OH) and halogenated my product. It does have an acrid smell and is dense and oily. It could be also the elimination product, definately not
water.
[Edited on 5-15-10 by rrkss]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Doubt it's the elimination product, the alkene's density should be 0,7 or a bit less, while the alcohol is 0,829. The standard test with Br2 would
indicate the presence of unsaturation. As a tertiary halide the bromide should react with aqueous AgNO3 on long contact.
I'd expect the alkene and alkyl bromide to be reasonable soluble in the mixed alcohols, unless there's a lot more ethanol; still I think it would
take some water as well to give 2 layers.
You really want a fractionating column to make sure of good clean separation, and distillation is the best route for cleaning it up.
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
I'm gonna dry it with cacl2 to remove ethanol and any residual water. Then do the distillation tommorow morning. My guess is that the pine smelling
stuff is my alcohol while the acrid stuff is the alkyl halide. Can't find any data on the alkyl halide though.
EDIT: The CaCl2, got warm and partially liquified when I added it to the product. Either it is picking up a ton of ethanol, or water or both. I
heard that CaCl2 forms adducts with alcohols, I hope that only applies to the lower alcohols.
[Edited on 5-15-10 by rrkss]
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Redid the distillation, and nothing came over at the boiling point of my product. Everything came over around 77 degrees.
I wonder if I made 2 butanone and did not have the reaction go completely to 3-methyl-3 pentanol. Never smelled MEK before but the product definately
does not smell like ethanol it has a very sharp odor.
As far as the appearance of my product, it is much clearer and colorless. Looks a ton better than it did.
I'm gonna put a drop in bleach and see if the haloform reaction runs to test my theory.
[Edited on 5-16-10 by rrkss]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Its unlikely you have any ketone product. If you only use 1eq. of grignard reagent, what you would get is 50% tertiary alcohol and 50% unreacted
starting material. Ketones are much more electrophilic than esters, and so would react far faster.
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Any ideas then what it could be? Does not smell like ethanol at all and boils at 77 degrees celsius. The only reagents used in the synthesis were
ethyl bromide, magnesium and ethyl acetate. The workup was done with 2 M H2SO4. I also dried it over CaCl2 to remove ethanol. I ran the reaction
using 2 equivs of EtBr and 1 equiv of EtOAc.
As of now, I see no sign of haloform reaction. Maybe it is my product and the boiling point information I have is wrong?
[Edited on 5-16-10 by rrkss]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by rrkss  | | Any ideas then what it could be? Does not smell like ethanol at all and boils at 77 degrees celsius. The only reagents used in the synthesis were
ethyl bromide, magnesium and ethyl acetate. |
I don't know what you made, but the BP of EtOAc is 77.
As we used to say in one lab where I worked "It's pure, whatever it is."
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Does not smell at all like ethyl acetate. Right now I am thinking of preparing bromine water to test for unsaturation since it failed the haloform
reaction test.
EDIT: Just made a bit of bromine water by dissolving NaBr in bleach and adding a drop of H2SO4. Added a drop of this to my unknown and it turned
yellow, then slowly faded back to colorless. I assume this is a positive test for unsaturation so I guess what I made is the elimination product: 3
methyl-2-pentene.
EDIT #2: The roundbottom distillation flask smelled a lot like the literary description of my product, however it was very charred and the little
remaining liquid was brown colored. Probably was the alcohol I was trying to seperate but decomposed before it could distill. If I rerun the
experiment, I will do the distillation under vacuum to to see if I get better results.
Right now my conclusion is that by overdoing the acid workup, I caused elimination to occur giving me a large amount of alkene. The literary value
for the alkene boiling point is 69 degrees celsius which is where a large portion distilled before I had a peak at 77 degrees. Then the remaining
actual alcohol started to decompose in the flask before a significant amount could boil and distill over.
[Edited on 5-17-10 by rrkss]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The ethyl acetate needs to be both quite dry and free of ethanol, otherwise your Grignard is going to react with those lovely H-O- groups to give you
ethane and reduce your yields - all the way to zero if there's too much wet stuff.
I'd suggest quenching with a solution of NH4Cl or (NH4)2SO4, afterwards adding acid dropwise until the pH drops to maybe 4. Wash with saturated NaCl
a couple of time, once with aqueous Na2CO3 or NaHCO3, then NaCl again. Preliminary dry with Na2SO4 if you have it, else MgSO4 but make sure it's
truly dehydrated. Only then attempt heating the product.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
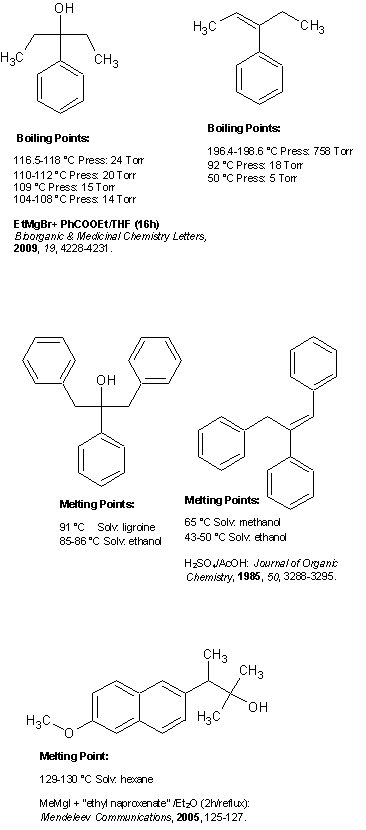
Rrkss, I assume you are practicing Grignards and you mentioned you have no means for a spectroscopic analysis. Therefore, I think you should perform
the addition of your ethylmagnesium bromide on esters that would give solid tertiary alcohols. This way you can purify them by recrystallization and
characterize by melting point. The problem is in that most common esters give liquid products with EtMgBr and I do not know what esters or other
proper carbonyl compounds you have access to. You should also consider the use of benzyl chloride for preparing the Grignard reagent. Using this gives
higher melting products, though the reaction with ethyl acetate would give 1,1-dibenzylethanol which has a mp of only 26-27°C. However the reaction
with benzoate esters would give a product with properly high mp. Even the dehydrated alkene product is still a solid at room temperature (see below).
For a solid product derived from EtMgBr and an OTC-like substrate you could react it with methyl or ethyl ester of naproxen. Only the Me derivative is
known in the literature and its mp indicates that the ethyl derivative should be a solid even though with a somewhat lower mp. You can also try with
ibuprofen esters, however the Me and Et derivatives are likely to be liquids due to the isobutyl group, but the Bn derivative should be a solid. Not
only you would be preparing products that can be purified without chromatography or fractionation, but you would additionally have the challenge of
preparing new compounds (and substrates for practicing other interesting transformations).
Also, keep in mind that you can commonly derivatize such tertiary alcohols into solid products by subjecting them to the Ritter reaction with
acetonitrile (in H2SO4/CH3COOH). The resulting products are secondary amides and are thus nearly always easily crystallizable solids. If you have no
acetonitrile, benzonitrile or other suitable nitriles, you can instead use urea as the nucleophile.
Attached is the information on ethyl and benzyl Grignard addition derivatives from benzoate esters, and the Me derivative from naproxene esters:
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Thanks nicodem for taking the time to write that, your post is a great help and gives me some new ideas  How would I go about seperating the Naproxin or Ibuprofen from the tablets in order to synthesize these esters? I'd
assume I would have to dissolve the pills in some solvent that does not dissolve the binders and then recrystalize it but have never done it before.
If there is some thread giving the details? If so, can somebody please point it to me because honestly I don't even know what to search for. How would I go about seperating the Naproxin or Ibuprofen from the tablets in order to synthesize these esters? I'd
assume I would have to dissolve the pills in some solvent that does not dissolve the binders and then recrystalize it but have never done it before.
If there is some thread giving the details? If so, can somebody please point it to me because honestly I don't even know what to search for.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I suggest to use naproxen, because the Et derivative from ibuprofen might not be easy to crystallize if solid at all at room temperature. Buy some
cheap naproxen pills with the smallest amount of additives, binders and colourants. Usually the content of these additives and binders is small
anyway. The sodium salt of naproxen is quite soluble in water, so you can make an acid/base extraction (particularly if you buy pills that already
contain the sodium salt - pay attention to this!) followed by recrystallization. Alternatively, if it is not the sodium salt a simple
recrystallization of the crushed pills might be enough (you check the purity by measuring the mp). You can also try a "solventless" acid/base
extraction (I would opt for this): dissolve the crushed pills in NaHCO3(aq) > vacuum filter off the insolubles > add some isopropanol or ethanol
to the filtrate (1/10 of volume) > precipitate the crude product from this solution by slowly adding 2M HCl while stirring and cooling > vacuum
filter and wash with plenty of water > dry > recrystallize. The solvents reported for recrystallization of naproxen are: toluene,
hexane/acetone, ethanol/water and chloroform/hexane. General esterification with H2SO4/EtOH should work well. Esterifications in refluxing MeOH are
often too slow, so stick to the ethyl ester (but I can later check the literature for a published method for both esters).
Good luck and keep us updated.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Purchased some Naproxen Sodium Salt at the grocery store for $5 for 50 220 mg tablets. Its very expensive for such a small quantity of reagent.
Every brand contained coloring agents so I ended up buying the generic brand which has
croscarmellose sodium, FD&C blue #2, macrogol, magnesium stearate, polyvinyl alcohol, povidone, pregelatinized starch, talc, and titanium dioxide
as its inactive ingredients.
I assume most of the binders will be insoluble in water while the naproxen sodium is so I will go for the acid/base extraction you mentioned.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Some of the additives are water soluble, others are not. I suggest doing a search on each and making lists of which is likely to end up where in the
extraction. You may decide to use a mixed water-alcohol extraction rather than a simple water one. It appears that using indigo to colour the
tablets blue is standard.
The price was a little high, a search shows 400 tablets for $15 down to $9.
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Reran the reaction, and this time quenched with ammonium sulphate. After the quench no longer released NH3 gas, I dissolved the NaBr into water, and
using a sep funnel seperated the product from the the aqueous layer.
Distilled off the ether/ethanol at normal pressure. Then distilled the organic layer at 55 torr and had a boiling range around 68-71 degrees. I
collected a colorless oily liquid with a stench similar to pines. With no other means of analysis I can only assume that I have the correct product
based on literary descriptions.
|
|
|