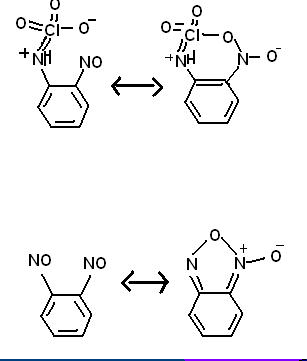
Stabilized Perchloro group
I designed a very bizarre compound, unusual in that it both stabilizes an organic perchloryl group and involves a chlorine atom inside and organic
ring. the resonance picture at the bottom is real, the top is hypothetical.
Benzene with an NHClO3 group on position #1 and a NO group on position #2. This configuration should share a resonance state with a ring of
-C-C-N-Cl-O-N-. While this seems difficult to imagine, I would like to point out that two adjacent NO groups on a benzene share a resonance with a
furozan/furoxan (the spelling varies) ring.
Analine reacts with dichlorine heptaoxide to give benzyl-NH-ClO3, where a positive charge sits on the N atom and an extra electron resonates on the
oxygen atoms in 3 of the 4 resonance states. There is a N-Cl bond with an order approaching two)
A perchloro group is more powerful of an oxidizing group than a nitro group for an explosive, however without an extra electron, like in the
perchlorate anion, the perchloro group becomes very unstable and the explosive is very sensitive to shock. Furozans are known to be particularly
stable because they form a (pentagonal) ring, thus increased stability could be expected from the perchloro froup in such a ring.
2 phenyl-NH2 + Cl2O7 --> analine perchlorate + phenyl NHClO3
|