Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
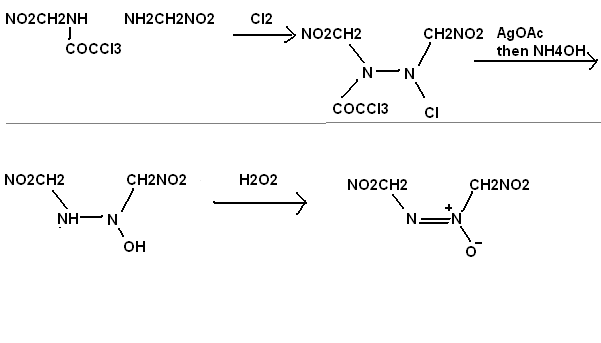
di[nitromethylene] diazo, N-oxide
Alternatively NH2CHNOH might react with a limited amount of very dilute H2O2 to make NO2CH2NNCH2NO2.
NH2CHNOH could probably be made by first using anyhydrous NH3 on formic acid to make NH2CHO, then adding NH2OH. I have made acetamidine from NH3 and
Acetic acid, maybe if I had used less NH3, might have got CH3CONH2. The oxygen balance on this compound (in picture) is good, should be more stable
than RDX.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I forgot, nitro methylene amine does not exist. One would have to start with nitroso methylene amine, then oxidize it to a nitro towards the end.
NH2CH2NO2 --> H+ -NHCH2NO2 --> H+ NHCH2 NO2-
NHCH2 disproportionates (in water) to hexamine and NH4OH.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Anders Hoveland  | Alternatively NH2CHNOH might react with a limited amount of very dilute H2O2 to make NO2CH2NNCH2NO2.
NH2CHNOH could probably be made by first using anyhydrous NH3 on formic acid to make NH2CHO, then adding NH2OH. I have made acetamidine from NH3 and
Acetic acid, maybe if I had used less NH3, might have got CH3CONH2. The oxygen balance on this compound (in picture) is good, should be more stable
than RDX.
|
So you have made CH3-C(-NH2)=NH from CH3-CO2H and NH3? But the question is how exactly?
To my knowledge it only results in CH3-CO2-NH4 and eventually under strong heating (dry distillation) to CH3-CO-NH2...eventually under very specific
conditions to CH3-C#N.
To my feeling NH2-CH=N-OH <==> NH2-CH2-N=O
and NH2-CH2-N=O would lead to:
-a polymer of the kind of NH2-(-CH2-N=N-)n-CH2-N=O
-a cyclic tetrazine ring of the kind CH2(-N=N-)2CH2
-a methylene reactant of the kind of diazomethane cyclo(-CH2-N=N-)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Or possibly NH2CHNOH would trimerize and give up NH3 in water to make [CH(NOH)]3N, somewhat similar to condensation of hexamine, but I doubt it. Or
maybe ([NH2]CN[OH])3 ring. This trimer could likely also be used as the precursor, as after oxidation it would hydrolyze to the desired product.
Formamidine can be found by a google search. NH2CH(NH)
I found this online too; "Formaldehyde oxime <--> nitrosomethane tautomerism"
Another thought: methyl mercuric perchlorate
PhHgNO3 is described online.
[Edited on 24-6-2010 by Anders Hoveland]
[Edited on 24-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  |
So you have made CH3-C(-NH2)=NH from CH3-CO2H and NH3? But the question is how exactly?
To my knowledge it only results in CH3-CO2-NH4 and eventually under strong heating (dry distillation) to CH3-CO-NH2...eventually under very specific
conditions to CH3-C#N.
To my feeling NH2-CH=N-OH <==> NH2-CH2-N=O
and NH2-CH2-N=O would lead to:
-a polymer of the kind of NH2-(-CH2-N=N-)n-CH2-N=O
-a cyclic tetrazine ring of the kind CH2(-N=N-)2CH2
-a methylene reactant of the kind of diazomethane cyclo(-CH2-N=N-) |
CH3CO2(-) NH4(+) should react with anhydrous NH3.
NH4OCOCH3 + NH3 --> NH4OH + H2NCOCH3
CH3CONH2 should be dehydrated and condense with more anhydrous ammonia.
CH3CONH2 + 2NH3 --> CH3C(NH)NH2 + NH4OH
Ammonia is a basic anhydride and can act like a dehydrating agent and condense.
NH3 and acetone condense to form a temporary imine
(CH3)2C=NH , but this quickly hydrolyzes back to the ketone in water, whereas guanidine does not.
Consider the structure of urea, to some extent there is
(-)O--C==N(+)H2
......... l
.........NH2
that is one of the reasons urea does not hydrolyze to ammonium carbonate.
Now NH2CH2NOH will almost certainly lose a NH3
Consider that NH2CH2NH2 does not exist in solution.
It would condense into hexamine and make NH4OH.
This can be prevented with protecting groups, like acetyl's, that prevent the hydrogens from ionizing of the Nitrogen atoms, which is the mechanism
for condensation.
For example, Ac2NCH2NH2 could likely exist in solution. To remove the Ac, just add a concentrated NH4OH to hydrolyze, forming the acetate.
[Edited on 2-7-2010 by Anders Hoveland]
|
|
|