| Pages:
1
2
3 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Here is what you tried to draw 
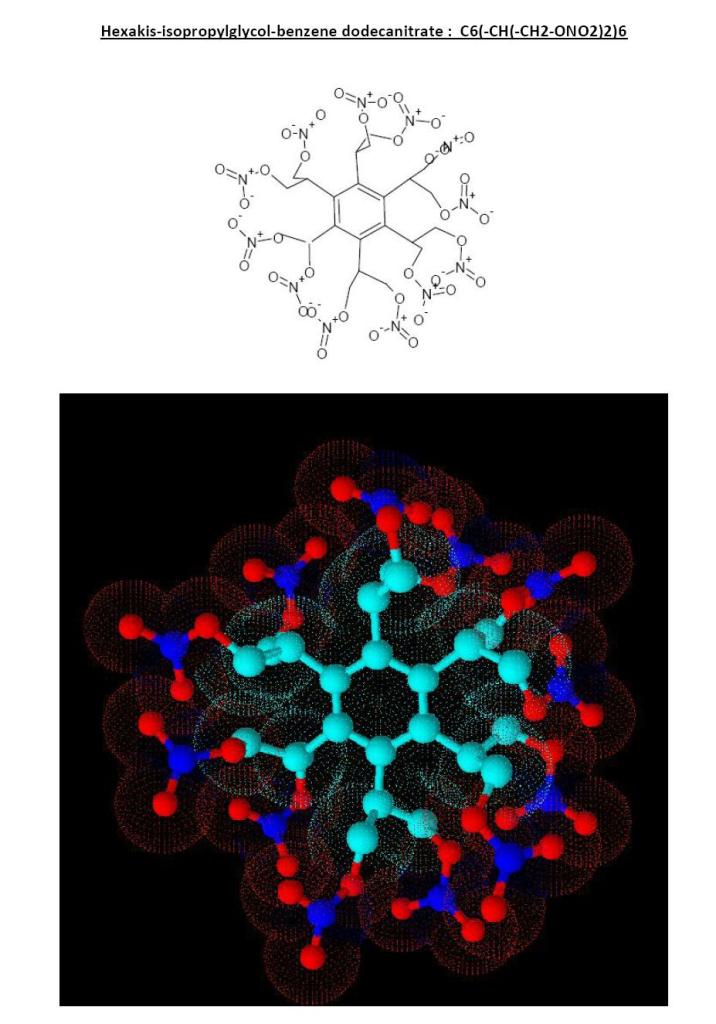
I think it is too bulky to be easy to do but who knows...experiment is the rule before rejecting.
Anyway it will be non ideal because of its negative OB (not enough O2 to burn all the carbon and hydrogen.
For some weird reason the hydrogens are lacking...they increases the bulky feeling...
[Edited on 25-6-2010 by PHILOU Zrealone]
[Edited on 26-6-2010 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
And here is what I was refering to slightly above 
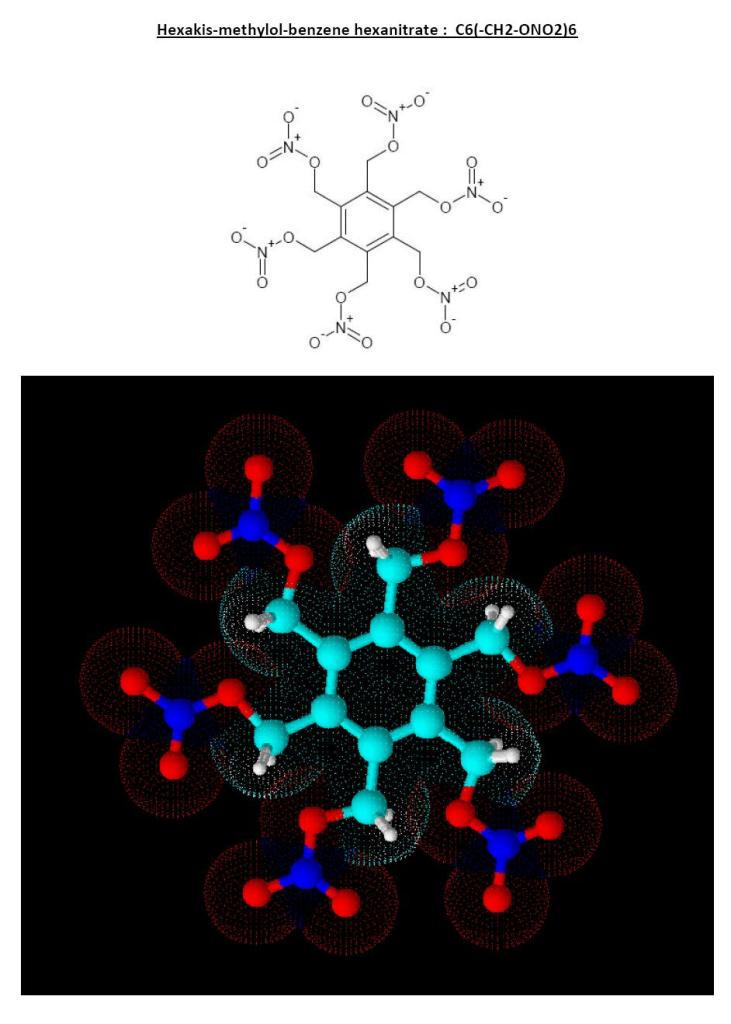
This compound exists and is also a bit on the negative side for OB...but stil possible if carbon monoxide is formed.
Maybe an extra oxygen provider would enhance its power!
[Edited on 25-6-2010 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Has anyone actually synthesized those two molecules, and lived to tell the tale?
[Edited on 25-6-10 by JohnWW]
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Zealone that was allmost EXACTLY what I tried to do!
Only difference being that propane dinitrate groups aren't paralel like l l l l l l,but more like l - l - l -.
Don't know if this is possible,but this might be natural aragement of the groups on the molecule if there is disturbance omong neighbour groups,you
can't really do anything about that,rather than observe it.
They are both beautiful!  
Nice idea to solve space problem with "hackenkreutz geometry"
(that is lousy translation,don't know to translate this into English, but I think you know what I mean)
OB is more like statistical than actual problem.
Actually it is more advantage than opposites.
For example TNT, you can add some significant mass by mixing with oxidants, while power of mix remains as pure explosive!
Some other explosives with negative OB, like some peroxides actually gain power, mass and stability by mixing them with oxidants (such as AN,KNO3...)
Another thing is war, all you want to do is to hurt enemy, so some CO can only be a good thing!
Stuff like these are way to exotic and powerful for civilian application where you need to take care of toxic fumes.
Only thing that might be tricky is their yield and sensitivity, but most phenyl nitrates are far from unstable.
If you have any documented and "high" yielding (relatively speaking) synthesis,please let us know!
[Edited on 25-6-2010 by mfilip62]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Hexakis methylolbenzene was synthetised by trimerisation of butyne-1,4-diol (HO-CH2-C#C-CH2OH); but this implies rare catalysts. The butyne glycol
itself can be synthetised from acetylen and formaldehyde; but it also require specific catalysts...
H-C#C-H + 2 CH2=O -cat 1-> HO-CH2-C#C-CH2-OH
3 HO-CH2-C#C-CH2-OH -cat 2-> C6(-CH2-OH)6
The butyne glycol could also lead to a powerful dinitrate ester but I wonder which of the two will be denser...the linear alcyne (richer at energy) or
the cyclic planar aromatic.
In theory the later, but in practice I don't know...
[Edited on 26-6-2010 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
If you find exact,step by step synthesis please PM me.
exotic catalists will be pretty much available for me in next few years. 
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Nitro compounds are much more insensitive than organic nitrate ester compounds. About as stable as nitro are organic amine perchlorates. Your
compound, though somewhat "oxygen defficient", would still be almost as powerful than if all the CO was oxidized to CO2. Yes, it is powerful, but
something more powerful and stable could be made with nitro groups instead, theoretically.
[Edited on 27-6-2010 by Anders Hoveland]
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
I bet this compound would be as powerful as HMX,at least, pretty dense and a little bit less stable than PETN.
Will it be solid or liquid!?
Only thing I am sure of is that OB is the least important thing to me, just add some AN and VIOLA!
Those prediction are solely based on my experience with similar stuff, and might be totally moronic
I don't have software to test theoretical crystal structure, density... nor did I ever attended higher organic chemistry.
(I left if to be my last class for few reasons, first after old kicks-off(he is VERY old and pain in the ass,so he probably met Franz Joseph in
person)
and after I solve things I hate like math and physics,so I can give full attention to my favorite class  ) )
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I did some more research. Apparently, 1mole quinone reacts with only 1mole NH2OH to make 4-nitroso phenol. You can see a picture of this compound
here: http://www.chemdrug.com/databases/dataimg/1/7729.png
This is still a way to get a nitroso on a benzene ring, so you can reduce it with HSO3- and get a --NHOH group that can be used to make energetic
perchlorate salts. This is just more speculation, but tell me if it seems unreasonable;
4-hydroxylamino phenol. reacts with acetyl chloride CH3COCl to put a protective acetyl group on the hydroxylamine instead of the hydrogen atom. A
nitration should first attack the ring. For example, nitration of TNT does not attack the methyl group first.
After the nitro groups are added, the acetyl is hydrolyzed off with NH4OH. This should leave, 2,5-dinitro, 4-hydroxylamino phenol, which could react
either as an acid or base. Adding HClO4 to this should make an explosive salt. It could also form a salt with Ammonia. Any comments welcome.
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Ammonia salt tends to be to too stable,and this doesn't look powerful,so iiiik...Not use of something too stable,expensive to make and not as
powerful.
Sounds like crack-head version of NH4NO3. 
Perchlorates tend to be relatively stable without presence of fuel,and this phenyl(organic fuel) perchlorate(strong oxidans) combination might be one
o the first useful phenyl derivatet primary explosives,who knows!? Lets do some experiments! 
Only problem that might be present is corrosion due the perchlorate,so not pretty much useful in military unless it unleashes deadly gasses like
phosgen(C+Cl+O quite likely!!!)
Imagine,High Explosive and chemical agent in one compund! That is what russians call GREAT SEXESS!!! 
I am curently searching for p-benzoquinoe which is next to imposible to find here.
(Yes, I know I can make it easily from p-hydroquinone,but it is hard to find too!)
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Phloroglucinol on wikipedia also has some interesting chemistry.
Making Benzoquinone
From Aniline using Potassium Dichromate
A solution of 20g of aniline in a mixture of water (600 ml) and concentrated sulfuric acid (160g, 80ml) is placed in a stout beaker immersed in
ice-cold water and continous stirring is begun. During the course of an hour 20g of finely powdered potassium dichromate are added in portions of
about 1g at a time, care being taken that the temperature does not rise above 10°C. A better method is to add a solution of 20g of sodium dichromate
in 100 ml of water from a dropping funnel. In either case the mixture is left in a cool spot overnight, and then a further quantity of potassium
dichromate (33g), or a solution of sodium dichromate (40g in 200 ml of water), added under conditions similar to the above. After four or five hours
the mass is extracted three times with ether, the ethereal solution dried with calcium chloride, and the ether removed by evaporation. The crude
quinone is purified by distillation in steam, or by sublimation, and forms orange-yellow needles with a characteristic pungent odour. Melting-point,
116°. Yield: 19g
[Sudborough & Campbell, Practical Organic Chemistry]
I am unsure if nitronium ions will spare the acetyl by attacking the benzene ring first.
"SO3" from H2S2O7 could also be used as the protecting group on the hydroxylamine.
C6H5N(OH)SO2(OH)
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Thanks!
Anyone tried this synthesis,an tips!?
Well,aniline is hard to find too,but I will try...
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Analine can be made by doing a nitration on benzene. The nitration bath must be 70C, just below benzene's boiling point. from wikipedia: "Nitrobenzene
is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid, called "mixed acid." Its production is one
of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (ΔH = −117 kJ/mol)."
Then zinc metal is added to nitrobenzene, and HCl solution is added. This reduces the nitro to an amine.
I think there is a more direct route to make benzoquinone from benzene or phenol, or benzoic acid, but it has a low yield and is messy. It involved a
severe oxidation, but I cannot remember the details.
There must be a way to buy quinone, perhaps an online photography store? A craft or big art store, in the photographic developer section?
[Edited on 29-6-2010 by Anders Hoveland]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Anders Hoveland  | | Aniline can be made by doing a nitration on benzene. The nitration bath must be 70C, just below benzene's boiling point. from wikipedia: "Nitrobenzene
is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid, called "mixed acid." Its production is one
of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (ΔH = −117 kJ/mol)."
|
I am very surprised that it should be so dangerous, because the nitration of benzene to C6H5NO2 was one of the class laboratory experiments that
students, including myself, were given to do in second-year Organic Chemistry at Victoria University Of Wellington, New Zealand, in 1967. I still
remember the strong, sickly smell of the product, like oil of bitter almonds. Another experiment that we later did on the product, in Physical
Chemistry, was to measure its dielectric constant.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Nitrobenzene is pretty easy to make here is a prep from the chemistry of powder and explosives,
"Preparation of Nitrobenzene. One hundred and fifty grams of concentrated
sulfuric acid (d. 1.84) and 100 grams of nitric acid (d. 1.42)
are mixed in a 500-cc. flask and cooled to room temperature, and 51
grams of benzene is added in small portions at a time with frequent
shaking. Shaking at this point is especially necessary lest the reaction
suddenly become violent. If the temperature of the mixture rises above
50-60°, the addition of the benzene is interrupted and the mixture is
cooled at the tap. After all the benzene has been added, an air condenser
is attached to the flask and the material is heated in the water
bath for an hour at 60° (thermometer in the water). After cooling, the
nitrobenzene (upper layer) is separated from the spent acid, washed
once with water (the nitrobenzene is now the lower layer), then several
times with dilute sodium carbonate solution until it is free from acid,
then once more with water, dried with calcium chloride, and distilled
(not quite to dryness). The portion boiling at 206-208° is taken as
nitrobenzene."
Benzene is pretty hard to nitrate, but can still be done pretty easily.
Also anders if you plan on making benzoquinone from benzene that will be a pain in the ass. The way I make benzoquinone is from the oxidation of
hydroquinone. I followed this procedure but modified it to use potassium chlorate instead of sodium chlorate, since I have a lot of potassium chlorate
from my electrolyis expiriments. It works very well and my yield is often around 90%, here is the page http://www.lycaeum.org/rhodium/chemistry/benzoquinone.html , there a whole lot on there. the chlorate method is high yielding and cheap.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
p-benzoquinone can be made from OTC precursors:
https://www.sciencemadness.org/whisper/viewthread.php?tid=82...
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Seems simple enough...
Anyone know how pure it is!?
Chlorinated byproduct might be a problem, how to remove it!?
This question might be better fitting into "techniques" but does anyone know how to purify it totally!?
Any method of simultaneous drying and resublimiation!?
[Edited on 30-6-2010 by mfilip62]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
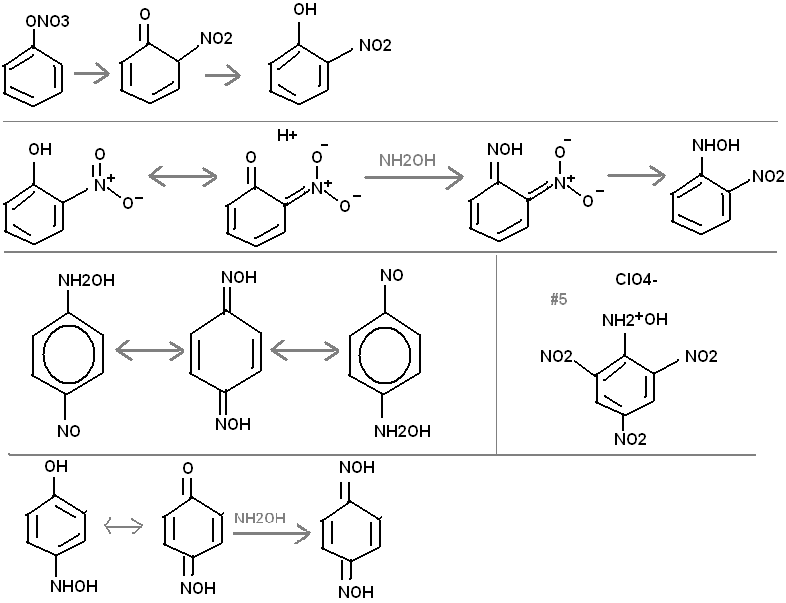
Bromine mixed with benzene and a little sunlight will quickly make bromobenzene, because of the radical cascade, discussed elsewhere on this forum.
Bromobenzene will react with AgNO3 dissolved in benzene to make benzene nitrate, which is unstable. This would be an interesting way to make
nitrophenol.
Here is an easy synthesis: Start with 2-nitro phenol. This should condense with hydroxylamine through the tautomeric ketone!
Whereas phenol would not condense with NH2OH; it would only form a salt. 1-hydroxylamino, 2-nitro benzene would form an explosive salt with HClO4.
Of course picric acid could be used instead, making compound #5.
Or you could start with dinitro phenol (the nitration generates almost entirely 2,4-dintro). Then do a nitrosation by adding to conc. H2SO4 and
slowly adding NaNO2. This will add an "NO" group in position 6. Use bisulfite to reduce the NO to a NHOH group. Then make the OH group in position 1,
condense with NH2OH, as previously described in this post. You will end up with 1,6-di[hydroxylamino], 2,4- dinitro benzene. This will of course form
a very powerful explosive salt with HClO4. The decomposition products from a molecule of this salt are:
3H2O, 2HCl, 5CO2, 1CO, 2N2. excellent oxygen balance.
The use of hydroxylamino groups, rather than plain amino, helps make this compound more powerful.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I wonder if you might have a hard time forming the perchlorate salt of trinitrophenylhydroxylamine because the electron withdrawing effect of the
nitro groups should reduce the basicity.. Picramide isn't basic from what I remember, and IIRC hydroxylamines tend to be even less basic than amines..
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Yes, it is not basic, but it should still be able to act as a base with a strong acid. Furthermore, even if the nitro groups do strongly pull
electrons away, an extra H+ ion should be able to stick on the nitro group.
I mean, if there is a positive charge on the NHOH group, then it will be double bonded to the ring, meaning one of the NO2 groups will also be double
bonded to the ring, each of its oxygen atoms having their own electron.
thus you would get a ==N(+)(O-)(OH) on the ring, and of course the ==N(+)H(OH) from the NHOH group.
The net effect still uses the NHOH as a base, except the hydrogen would go on a nitro, not the hydroxylamino.
Hope this makes sense, just do not feel like making another picture.
Of course, a perchlorate salt of trinitro-analine would be quite acidic and deliquescent (it would have to be kept from absorbing moisture from the
air), and it probably would not crystallize out of solution easily. Mono-nitro-Analine could be dried much more easily; and probably could be handled
with bare hands without chemical burns.
Also just to clarify:
Obviously nitro-phenol does not condense with NH4OH, it only forms the ammonium salt.
Obviously phenol would not condense with hydroxylamine, it would only form the carbolate salt.
It is mono or di-nitro phenol that should condense with NH2OH, since hydroxylamines are known to condense with ketones to make oximes.
This is a synthesis most members here can do easily, and it would be an interesting reaction.
[Edited on 2-7-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
[Edited on 6-7-2010 by Anders Hoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
CL-14 from Rosorcinol
I did a search and could not find anything about the explosive Cl-14 in this forum. Not wanting to start a new thread, I will just put a post here,
since the proposed chemistry is similar.
Here is some information about Cl-14,
http://www.digitalprecursor.org/roguesci/chemlab/energetics/...
detonation velocity 8050 m/sec.
Rosorcinol (1,3-dihydroxy-benzene) can be converted to phloroglucinol (1,3,5-trihydroxy-bezene) by fusion with solid sodium hydroxide. the reaction
also releases hydrogen gas.
"THE LIBERATION OF HYDROGEN FROM CARBON COMPOUNDS", Shipley Fry, Else L. Schulze, Helen Weitkamp
J. Am. Chem. Soc., 1924, 46 (10), pp 2268–2275
This preparation of phloroglucinol is of interest since the substance can tautomerize into the tri-ketone of cyclohexane http://en.wikipedia.org/wiki/Phloroglucinol , and thus condensation of phloroglucinol with NH3 would result in 1,2,3-triamino-benzene. This could
then potentially be nitrated [see comments at bottom of this post] into TATB, http://en.wikipedia.org/wiki/TATB
The TATB would finally be treated with aqueous chlorine for conversion, in moderate yield, into the "advanced" explosive CL-14
(5,7-diamino-4,6-dinitrobenzofuroxan);
For example, the reaction of 2-nitroaniline with hypochlorite solution produces "benzofurazan oxide" (more commonly named benzofuroxan).
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4...
| Quote: |
The literature extensively describes the preparation of phloroglucinol by hydrolysis of 1,3,5-triaminobenzene in the presence of concentrated
hydrochloric acid.
hydrolysis of 1,3,5-triaminobenzene to give phloroglucinol, mention may be made of U.S. Pat. No. 4,115,451. That patent recommends hydrolysis of
1,3,5-triaminobenzene in an excess of concentrated hydrochloric acid at a temperature of 100 to 200° C., to end up with phloroglucinol. This
hydrolysis step is followed by a step of extraction with an acetic ester. The extracted phase containing the phloroglucinol crystallizes after
cooling.
|
My guess is that the reaction is reversible, but that such a reaction would not be mentioned in patents because phloroglucinol is industrially much
more expensive than triaminobenzene.
As for the nitration, I also found this
| Quote: |
The triethyl tricarbamate of 1,3,5‐triaminobenzene was prepared... The tricarbamate underwent nitration to give the mono‐, di‐, or
trinitro analogs in excellent yield
|
suggesting that direct nitration of unprotected 1,3,5‐triaminobenzene may be problematic. Probably best to react with acetyl chloride first.
After the nitration, the acetyl (or carbamate groups resulting from oxidation) can be hydrolysed off by simple treatment with 20% solution of NH4OH.
[Edited on 6-12-2011 by AndersHoveland]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
References ?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
| Quote: |
From the foregoing results, it appears that phloroglucinol, a trihydric phenol, reacts more energetically with aromatic amines than the dihydric
phenols [such as 1,3-dihydroxybenzene], which in turn are more reactive than the monhydric compounds [example, phenol].
In view of the complete analogy between the action of ammonia and the substituted amines, it seems probable that phloramine, the product of
the action of ammonia on phloroglucinol, is also a derivative of trihydroxybenzene, and not of the secondary phloroglucinol, as indicated by
Baeyer (Abstr. 1886, 350).
|
Journal of the Chemical Society, Volume 60
(Great Britain), p191
| Quote: |
Phloramine- A compound produced by the action of ammonia on phloroglucin.
C5H6O3 + NH3 --> C6H7NO2 + H2O
|
A dictionary of chemistry and the allied branches of other sciences, Volume 4, p488
It would appear that whereas phloroglucin[ol] is the tautomer of 1,2,3-trihydroxy-benzene, phloroglucin, which is a partial imine of a tri-ketone, is
the tautomer of 3,5-dihydroxy-aniline.
| Quote: |
The conversion of phenol into aniline proceeds under very drastic conditions (350-450°C, 50-60 bar) and the substitution of one hydroxyl group in
resorcinol by an amino group
occurs quite readily at 200°C, whereas phloroglucinol gives 3,5-dihydroxyaniline and 3,5-diaminophenol in almost quantitative yield
under very mild conditions (long storage at room temperature with ethanolic solution of ammonia).
It was [calculated] that the enolic form, 1,3,5-benezenetriol, is by far more stable than the keto form, 1,3,5-cyclohexanetrione. On the other hand,
the latter is more abundant in the phloroglucinol system than is the keto form of phenol (2,4-cyclohexadien-1-one)
|
The picture (on page 718, figures 15 and 16) clearly shows that hydroxylamine condenses with phloroglucinol to form the tri-oxime of
cylcohexane.
Chemistry of Phenols, Part 2
Zvi Rappoport, p717-718
http://books.google.com/books?id=0pVQgwt5ODoC&pg=PA718&a...
I cannot find any references about further condensing 3,5-diaminophenol with ammonia to form 1,3,5-triaminobenzene, although I did find a reference to
the reverse reaction,
| Quote: |
The most convenient method of preparation is the hydrolysis of the hydrochloride of 1:3:5-triamino- benzene by prolonged boiling with water.
|
Chemistry of carbon compounds: a modern comprehensive treatise, edited by E. H. Rodd, p483; specific reference made to H. Weidel and J. Pollak,
Monatsh., 1900, 21, 20
| Quote: |
hydrolyse 1:3:5-triaminobenzene with 5% HCl and obtain 3:5-diaminophenol.
|
Reports on the progress of applied chemistry, Volume 33, p74
It may be possible that a third amino group cannot be added. But the fact that hydroxylamine can fully condense with 1,3,5-trihydroxybenzene suggests
that ammonia may also be able to react likewise.
Summary of this Post
The above post is essentially providing references about the replacement of hydroxyl groups by amine groups. Ammonia can easily condense with certain
specific types of hydroxybenzene derivitives through the formation of a transient imine, =NH, on the ketone, =O, tautomers. This is normally the type
of information found in the organic chemistry section, but I think this type of basic organic chemistry is important in the energetics section also.
[Edited on 6-12-2011 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Limited oxidation of para-quinone-di-oxime, HON=C6H4=NOH, produces para-di-nitroso-benzene,
O=N-C6H4-N=O
| Quote: |
...ortho-dinitroso-benzene, a para-isomere of which had already been made known by Nietzki. It was produced by the oxidation of para-quinone-dioxime.
|
Report of the annual meeting, Volume 64, Part 1894, p620
British Association for the Advancement of Science, year 1894
|
|
|
| Pages:
1
2
3 |
|