| Pages:
1
2
3 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I remain unpersuaded 
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I have proved that at least two of the hydroxy -OH groups can be replaced with amine -NH2 groups. Just have to find information
proving that third hydroxyl group can likewise substitute off also.
But you must admit it is remarkable that simple condensation with ammonia can effect such a substitution.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I can see a halogen being exchanged or a nitro being reduced to get the amino,
a lot easier than I can see the ammonium salt of a phenolic acid being thermally dehydrated to the amino. That was a new one on me and I strongly
suspected it would be a less than convenient method, probably not occurring easily and possibly not occurring at all.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
C-14 from mothballs
I also have another similar idea that Cl-14 could be made from paradichlorobenzene. When I recently went to the store, I was surprised to see that
relatively pure paradichlorobenzene is still used in some brands of mothballs.
Nitration of paradichlorobenzene
http://books.google.com/books?id=NUo2AQAAIAAJ&pg=PA2260&...
Here is a graph which show that the paradichlorobenzene is mostly consumed after 15 minutes using 12M (molar concentration) HNO3, at only 10 degC.
http://www.chem.uiuc.edu/weborganic/arenes/Nitration/diClben...
http://www.chem.uiuc.edu/weborganic/arenes/Nitration/diClben...
Obviously it would take longer to add the second nitro group.
"Both 2- and 4-chloronitrobenzene react with anhydrous ammonia at 200degC to form the corresponding nitroaniline, whereas 3-chloronitrobenzene did not
react under these conditions."
V. A. Tarasevich, M. F. Rusak, A. B. Tereshko, and N. G. Kozlov, Zh. Obshch. Khim. 67, 671 (1997); Chem. Abstr. 128, 2701275r (1998)
In the presence of the iodide ion, dry NH3, dissolved in pure alcohol, reacts rapidly with 2- and 4-chloronitrobenzene at 100degC.
1-chloro-2,4-dinitrobenzene reacted with alcoholic ammonia at room temperature.
V. A. Tarasevich, M. F. Rusak, A. B. Tereshko, and N. G. Kozlov, Zh. Obshch. Khim, 67, 457 (1997); Chem. Abstr., 128, 270275r (1998)
Essentially 1,4-dinitro-2,5-diaminobenzene will be obtained, which could then be reacted with hypochlorite to form the di-furoxan of benzene. From
here you might be wondering how this will ever get to Cl-14, but it is actually quite easy. Just do a nitration again.
Nitrobenzofuroxans can tautomerize in very remarkable ways, see "scheme 17" on page 16
4-methyl,3-nitro-benzofuroxan is thus the same compound as 6-methyl,3-nitro-benzofuroxan.
http://www.ark.chem.ufl.edu/Published_Papers/PDF/256.pdf
So once there are adjacent nitro groups, those furoxans can essentially move around on the molecule.
Finally, just reduce one of the nitro groups to an amine group using a ferrous salt (FeCl2), similar to reducing picric acid to picramic.
| Quote: |
When solutions of picric acid and Ferrous Sulfate are mixed, no obvious reaction happens, but when the Iron is precipated by addition of a strong
base, the liquid takes on a deep red color, and Ferric Hydroxide precipitates. Ammonia may be used as the base, in which case, addition of acetic acid
to the filtered solution (the insoluble Ferric Hydroxide being previously completely filtered out) causes thin red needle crystals of picramic acid to
precipitate out. This way is much better than using sulfides and will give a much superior product to the previous one. (researcher Aime Girard)
|
The final product should be Cl-14 (5,7-diamino-4,6-dinitrobenzofuroxan)
......................................................................................
A note for terminology: In the benzofuroxan molecule, the furoxan ring is attached to the 1- and 2- positions of the benzene ring. The
mention to "di-furoxan of benzene" in htis post referred to the two furoxan groups attached in the 1,2- and 4,5- positions.
I will post more information, more supporting references, and diagrams of this idea later. If someone else wants to make a diagram of this idea,
showing the molecular structures, please feel free to save me the trouble. 
[Edited on 7-12-2011 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Third Idea for Route to Cl-14
Here is a third idea for a new synthesis route to Cl-14,
see the attachments at the bottom of the page on this site:
http://sites.google.com/site/energeticchemical/furoxan-witho...
(I made a mistake in the picture- nitration of aniline produces mostly 3-nitroaniline, best to start with chlorobenzene instead, partially nitrate it,
then react with NH3 in anhydrous alcohol)
| Quote: |
Synthesis for Nitrating Chlorobenzene
90 mL of chlorobenzene is added dropwise with a dropper pipet or buret to a previously prepared, and cooled to room temperature, mixture of 110 mL of
99% nitric acid and 185 mL of 99% sulfuric acid, in a 1000-mL beaker, while the mixture is stirred mechanically with a magnetic stirrer. A stirrer is
essential for the length of time required, you may try this by hand with a stirring rod at your own risk. The temperature will rise because of the
heat of the reaction, but should not be allowed to go above 50-55 °C. After all the chlorobenzene has been added, the temperature is slowly raised to
95 °C and is kept there for 2 hours longer while the stirring is continued. An upper layer of light yellow liquid solidifies when cold. The layer is
removed, broken up under water, and rinsed. The spent acid, on dilution with water, will precipitate an additional quantity of dinitrochlorobenzene.
All the product is brought together, washed with cold water, then several times with hot water while it is melted, and once more with cold water under
which it is crushed. Finally, it is drained and allowed to dry at room temperature. The product, melting at about 50 °C, consists largely of
2,4-dinitrochlorobenzene, along with a small quantity of the 2,6-dinitro compound, m.p. 87-88 °C. The two substances are equally suitable for
manufacture of other explosives or alone as an explosive. You will need a graduated cylinder for measuring liquids, and a thermometer to monitor the
temperature.
Warning: Dinitrochloro benzene is extremely poisonous. Inhaling the vapors can be deadly, as the compound oxidizes hemogoblin in the blood to a
form that cannot bind with oxygen, leading to ischemia. Skin contact causes severe rash, itchy burning sensation, and blistering, analogous poison
ivy.
|
2,4-dinitroanaline undergoes an unexpectedly complex reaction when treated in alkaline methanol solution at 50degC with aqueous sodium hypochlorite.
The product is a “chloromethoxybenzofurazan oxide”, namely 1-chloro-3,4-furoxan-4-methoxy-benzene.
Green and Rowe (1912). and
“Furazan Oxides. An Unusual Type of Aromatic Substitution Reaction", Frank B. Mallory, Suzanne P. Varimbi (1963)
As can be seen in the second attachment (in the site), the chlorine and methoxy groups could potentially be substituted by amine groups by react with
NH3.
Here is another reference to an adjacent amino and nitro group on a benzene ring being converted to an adjoining furoxan ring by treatment with NaOCl,
Green and Rowe, J. Chem. Soc., 101, 2452 (1912).
A fourth idea for a route to Cl-14
Not related to the previous idea, I also found a source that states that aniline reacts with bromine water, without a catalyst, to form
2,4,6-tribromo-aniline.
"Nitrations of Aromatic Compounds"
(Microsoft Powerpoint)
This type of reaction could potentially be very useful, because bromine substitutes off far more easily than chlorine atoms. Reacting the product with
anhydrous NH3 would no doubt produce 1,2,4,6-tetraminobenzene. Protect the amine groups by reacting with acetic anhydride, then perform a nitration.
React with NH4OH to hydrolyse off the acetyl groups. The resulting 3,5-dinitro-1,2,4,6-tetraminobenzene might be a useful precursor to make Cl-14.
| Quote: |
Azobenzene is also produced when KMnO, acts on aniline, or when aniline is oxidized in alkaline solution by hypochlorite.
|
Proceedings of the American Pharmaceutical Association ,Volume 58, p288
So reaction of the 3,5-dinitro-1,2,4,6-tetraminobenzene with sodium hypochlorite solution could potentially result in
azo-bis[nitrobenzo-di-furoxan], which is essentially two molecules of Cl-14 that are joined together by the former amino groups, the new molecule no
longer containing any hydrogen atoms.
(N2O4)2(NO2)C6-N=N-C6(N2O4)2(NO2)
Azo linkages are easily reduced to hydrazo linkages, -NH-NH-
So I suppose this compound could be regarded as the "di-Cl-14"! 
Bromine water also similarly reacts with phenol to give tribromophenol.
Journal of the Chemical Society, Volumes 31-32, p361
| Quote: |
Chloro and bromobenzene reacted with the very strong base sodium amide (NaNH2 at low temperature (-33 ºC in liquid ammonia) to give good yields of
aniline (aminobenzene). However, ortho-chloroanisole gave exclusively meta-methoxyaniline under the same conditions.
|
source: http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/benz...
Anisole is the same thing as methoxybenzene, CH3-O-C6H5
[Edited on 8-12-2011 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I found some more interesting information that may possibly relate to the condensation products of NH2OH on quinone:
| Quote: |
β-Phenylhydroxylamine forms colourless needles, m.p. 81°-82° ; soluble in 10 parts hot and 50 of cold water, readily soluble in alcohol, ether,
carbon disulphide, and chloroform, sparingly in petroleum. It dissolves in sulphuric acid with a deep blue colour. By heating at 100° azobenzene
together with aniline, azoxybenzene, and other products are formed. Oxidation with potassium permanganate gives first nitrosobenzene, then nitrogen
and azoxybenzene (Bamberger and Tschirnmer, Ber. 1899, 32, 342); in dilute neutral solution hydrogen peroxide yields azoxybenzene, in alklaline
solution azoxybenzene and nitrobenzene (Bamburger, Ber. 1900, 33, 119). In the presence of hydroxylamine and air it is partly oxidized to azoxybenzene
and partly reduced to aniline, phenylazoimide, and benzeneazohydroxyanilide also being formed… Mineral acids yield p-aminophenol and azoxybenzene;
alcoholic sulphuric acid gives azoxybenzene, o- and p-phenetidine, o—and p-aminophenols, aniline, and other compounds.
|
"A dictionary of applied chemistry", Sir Thomas Edward Thorpe, p88
Terminology and Structures
Just to save you the trouble of having to look up all the structures, I made a glossary of terminology below:
"beta-phenylhydroxylamine" refers to a benzene ring connected to the nitrogen atom of hydroxylamine.
C6H4-NHOH
as can be seen in the picture below,
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1...
the structure for "phenetidine" is NH2-C6H4-O-CH2CH3
(obviously ethanol was used as the alcohol in the reaction)
"phenylazoimide" is the same thing as phenyl azide,
C6H5-N=N=N
azoxybenzene is C6H5-N=N(O)-C6H5, with the oxygen atom bonded to one of the nitrogen atoms.
azobenzene C6H5-N=N-C6H5
I think "benzeneazohydroxyanilide" might be
C6H5-N=N-C6H4-OH
[Edited on 8-12-2011 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Here is a diagram, starting with paradichlorobenzene.
The final product is essentially Cl-14 but without the amino group.
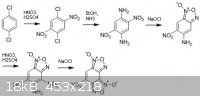
I am not entirely sure what the result of treating NaOCl with 1,4-dinitro-2,5-diaminobenzene would be. I suspect two furoxan rings cannot both form in
these positions. If only one furoxan ring forms, it might be possible that the other amino group could be oxidized, although it would be more
resistant to oxidation because it is in an electron-donating position. Perhaps best to use only a limited proportion of hypochlorite solution to avoid
potential oxidation of that second amino group.
[Edited on 8-12-2011 by AndersHoveland]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
There is a big chance your paradiamino-2,5-dinitrobenzene will be oxydised into a 2,5-dinitroparaquinon...
H2N-C6H2(NO2)2-NH2 -ox-> HN=C6H2(NO2)2=NH --> O=C6H2(NO2)2=O + 2NH3
On the other hand amino groups in ortho position of a nitro group on an aromatic ring often undergoes thermal oxydoreduction...into furoxan without
need for NaOCl.
It is the very case for 1,3,5-Triamino-2,4,6-trinitrobenzene what turns upon heating into a mono, di or trifuroxan derivative...what explains the very
high stability of related compounds and use in LOVA HE.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
That could in fact be the case. Thanks for this insight.
Paradiaminobenzene, for example, hydrolyses to hydroquinone under mildly acidic conditions.
On the other hand, the two electron-withdrawing nitro groups, which are in the ortho- position relative to an amino, would be expected lend stability
towards the configuration. Apparently 1,4-diamino-2-nitrobenzene is also used in hair products (where it hydrolyses to the quinone), which is not
encouraging. An acetyl protecting group on one of the amines might prevent hydrolysis/oxidation so that the other amino group could be oxidized to a
furoxan. Indeed, the industrial production of 1,4-diamino-2-nitrobenzene involves acetylation (with Ac2O) of both the amino groups before nitration.
2,5-dinitroparaquinone is described in the literature as not being very chemically stable.
[Edited on 24-1-2012 by AndersHoveland]
|
|
|
| Pages:
1
2
3 |
|