Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
oxy-nitrogenous perchlorate explosive
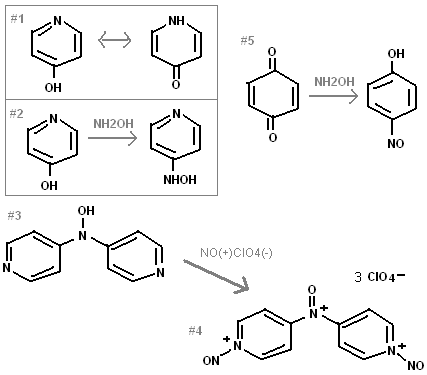
I designed a new energetic compound, that is unique in that it is a perchlorate salts with oxy-nitrogenous bases.
Benzoquinone is known to condense with hydroxylamine to make para-Nitrosophenol (figure #5). 4-nitrosophenol is also called an oxime, even though no
NOH group exists because "quinone 4-oxime" converts to this compound.
I think that two molecules of 4-hydroxy pyridine will likely condense with one NH2OH to make a bi(pyridyl)hydroxylamine compound #3. Compound #3 will
act as a base toward nitrosyl cations. The R2NOH will get oxidized to a R2N(+)O. Nitrosyl perchlorate can be made by bubbling nitric oxide and
nitrogen dioxide together into concentrated perchloric acid.
3HClO4 + NO + NO2 --> 2(NO+)ClO4(-) + (H3O+)ClO4(-)
The mono and dihydrates of perchloric acid are solid. Anhydrous perchloric acid is a liquid.
Compound #4 should be very powerful and insensitive, having an excellent oxygen balance. It is interesting that it has no nitro groups and no amines
to act as bases for the perchlorate anions.
I think that 4-hydroxy pyridine will condense with NH2OH through an intermediate quinone. (#1,#2) This type of chemistry for quinones is quite common.
1,3,5-hydroxy benzene is known to react as a triple ketone in many reactions.
This explosive, although relatively insensitive, would not be chemically inert. It would be highly acidic, and violently hydrolyze with water.
[Edited on 29-6-2010 by Anders Hoveland]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: | | I think that two molecules of Benzoquinone will likely condense with one NH2OH to make compound #3 |
I think you are mistaken.. I think you must mean it would make di(nitrosophenyl)hydroxylamine not di(pyridyl)hydroxylamine as the picture shows. I'm
sure this is a typo..
#4 looks like an interesting explosive, but the fact that you have to deal with making nitrosyl perchlorate and acquiring 4-pyridone is kind of a buzz
kill. Also the OB looks too poor for it to be really poweful. Although you might be able to add some nitro groups to the pyridines to increase the OB
and power.
[Edited on 29-6-2010 by 497]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I meant that
"4-hydroxy pyridine will likely condense with one NH2OH to make a bi(pyridyl)hydroxylamine compound #3."
If less hydoxy pyridine is used, 4-hydroxylamino pyridine will likely result.
A google search came up with "interaction energy of water with the tautomeric species 4-hydroxy-pyridine". There is a chemical supplier: http://www.capotchem.com/en/1116.htm
This compound is apparently not impossible to come by.
One site reported that the book, W. Theilheimer. Synthetic Methods of Organic Chemistry. Volume 7, 1953, p. 181,
contained a synthesis for 4-hydroxy pyridine.
"4-Hydroxypyridine is hazardous since it can irritate eyes, skin, lung, and gastrointestinal tract" Melting point 148 ºC
Boiling point 230-235 ºC
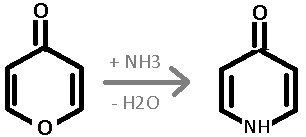
I found a picture on wikipedia, which I am including in this post. I think anhydrous NH3 is used to dehydrate and add ammonia. Here is the wikipedia
article in German: http://de.wikipedia.org/wiki/4-Pyridon
The translation is: "Analogous to the isomeric 2-pyridone can 4-pyridone by reaction of 4-pyrone with ammonia are obtained. The keto form 4-pyridone
is in chemical equilibrium with the tautomeric Hydroxoform 4-hydroxypyridine." It mentions that the ketone is the dominant form in solution. It forms
a yellow complex with FeCl3.
4-chloropyridine can be made with pyridine N-oxide and thionyl chloride SOCl2, making 4-thiopyridine, which then reacts with chlorine to make
4-chloropyridine.
The direct chlorination of pyridine will mostly result in almost entirely 2-hydroxy pyridine, with some 4-hydroxy byproduct.
My German is not good, so I may have mistranslated.
Bromopyridine is likely to react with AgNO3 to make hydroxy pyridine, as the nitrate will quickly hydrolyze in solution.
A patent that contains information about 2-hydroxy pyridine, which could possibly be used instead of 4-hydoxy pyridine in my reaction, can be found:
http://www.freepatentsonline.com/2752356.pdf
The wikipedia article in English for 2-pyridone is: http://en.wikipedia.org/wiki/2-Pyridone.
Hopefully this is more than enough information to make 4-pyridone, which is just another name for the same compound.
The German chemical articles on wikipedia seem much more extensive than the English ones. I think the Germans have a stronger tradition in chemistry.
[Edited on 30-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 497  |
#4 looks like an interesting explosive, but the fact that you have to deal with making nitrosyl perchlorate and acquiring 4-pyridone is kind of a buzz
kill. Also the OB looks too poor for it to be really poweful. Although you might be able to add some nitro groups to the pyridines to increase the OB
and power.
|
Yes, the precursor would be quite difficult to come by, but the NOClO4 should not be too difficult to make. Several videos using it as an explosive
mixture with nitromethane are posted on youtube. Also, I forgot to mention, only a limited ammount of nitrosyl perchlorate should be used, otherwise
excess NO+ might add NO groups to the rings.
You said the oxygen balance did not look good. Did you notice the "3ClO4-" that accompanied compound #4 ? That means there are 3 perchlorate anions
for every cation.
I have posted a much easier synthesis in "quinone explosives" topic, that starts with phenol. This is also quite interesting, and should be more
suited to members here.
[Edited on 2-7-2010 by Anders Hoveland]
|
|
|
|