turbo hauser base effect on some compounds
i was reading about turbo-hauser bases
and that common ones are
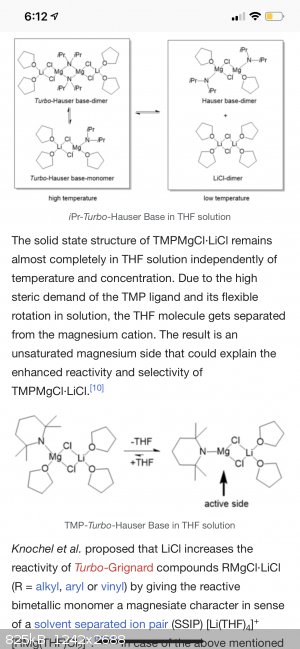
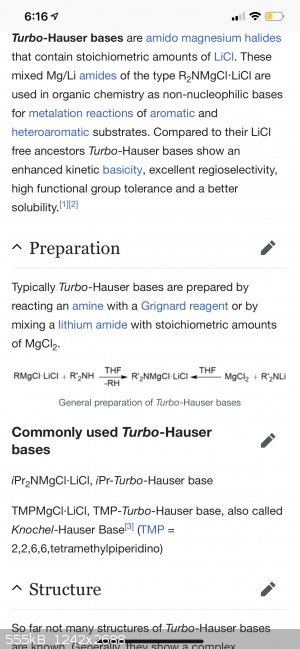
iPr2NMgCl·LiCl, iPr-Turbo-Hauser base
TMPMgCl·LiCl, TMP-Turbo-Hauser base
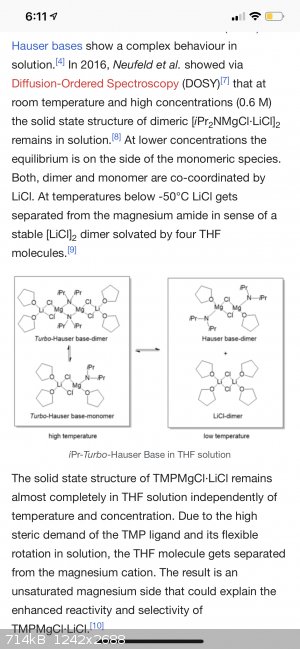
i also read that those two differ in their reactions
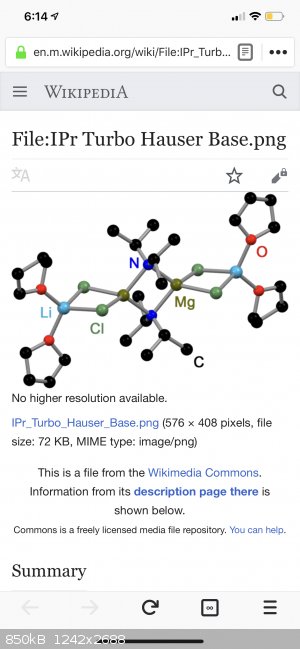
[the 9th picture down (here)]
https://en.m.wikipedia.org/wiki/Turbo-Hauser_bases
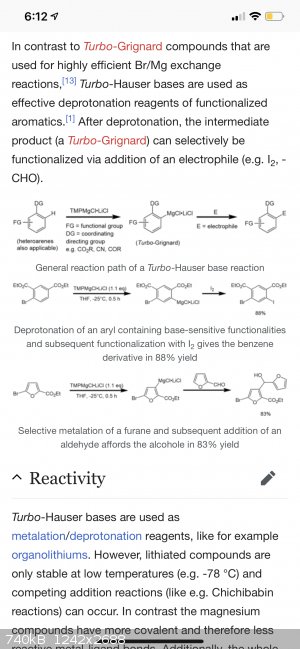
“iPr2NMgCl·LiCl can show a different reactivity compared to TMPMgCl·LiCl. Armstrong et al. showed that the TMP-Turbo-Hauser base easily metalates
ethyl-3-chlorobenzoate in the C2 position, while the same reaction carried out with the iPr-Turbo-Hauser base resulted in no metalation at all.
Instead, an addition-elimination reaction occurs.[5]”
[5] Armstrong D. R.; García–Álvarez, P.; Kennedy, A. R.; Mulvey, R. E.; Parkinson, J. A. (2010). "Diisopropylamide and TMP Turbo-Grignard
Reagents: A Structural Rationale for their Contrasting Reactivities". Angew. Chem. Int. Ed. 49 (18): 3185–3188. doi:10.1002/anie.201000539. PMID
20352641.
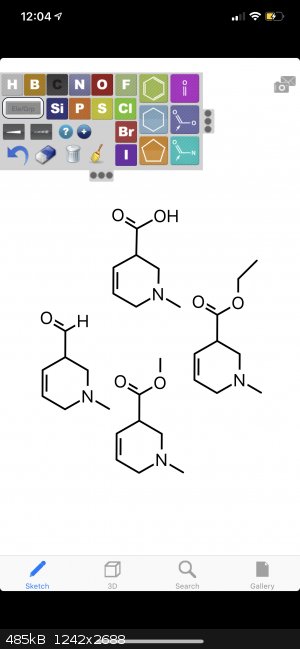
i noticed that it addition-substitution reacted with an ethyl ester, so i was wondering if it would replace
1) OH acid
2) H aldehyde
3) OMe methyl ester
i assume it would work better with the OH, (but what about the aldehyde?)
i was then wondering if it was only directed because of the 2-Chloro,
but anyway, the two substitutions that i am wondering about are regarding
the idea of Et2NMgCl•LiCl substituting the OH in the attached molecule and also in the case of m-Toluic acid (producing DEET in that case).
i was wondering if it would preferentially metallate the molecules where there are pi orbitals at the double bonds.
[Edited on 28-2-2019 by pomegranate]
[Edited on 28-2-2019 by pomegranate]
|