Abdibsz
Harmless

Posts: 8
Registered: 9-6-2019
Location: Mercer Island, WA, USA
Member Is Offline
|
|
Electrolytic production of NaOH from salt water - Troubleshooting
Hey, this is my first post so I'm not sure if this in the correct section. Anyways, I'm trying to produce NaOH through the electrolysis of salt water.
I'm pretty sure it'd be easier for me to just buy some but that's not an option for me right now. I've attached a diagram of my setup.
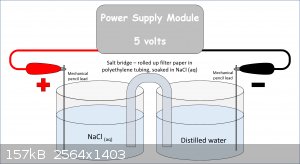
The thing is, it's not working. I think it's because of how terrible a conductor distilled water is. What can I add to the water to make it conductive
without drastically lowering the purity of the resulting NaOH solution by too much?
P.s. Sorry for the crap diagram, as you can tell, art isn't my strong suit Lol
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Add some NH3(aq)? It has a little bit of NH4+ and OH- so it can help conduct electricity, and you can remove it afterwards by heating and driving off
the NH3 gas.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Well you could move the electrode a lot closer to the bridge to get the resistance down.
Or why not dissolve a little NaOH in to begin with.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
|
|
|
Abdibsz
Harmless

Posts: 8
Registered: 9-6-2019
Location: Mercer Island, WA, USA
Member Is Offline
|
|
Thanks! I'll try moving the electrodes and adding ammonia as soon as I get my hands on some. I think the QFC near my house sells some ammonia but I
think it contains surfactants. Do you think it's worth the trouble to purify it or would it be easier to continue searching for some without
surfactants?
[Edited on 6/9/2019 by Abdibsz]
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Well, the thing with your setup is this: once you have some Na+ ions transferred across the resistance will go down. So, it won't stay bad forever.
But you will have to be patient -- electrolysis takes time.
You are also going to have NaCl diffuse across the salt bridge eventually. So you are not going to have amazing purity. You could probably add a
little salt to your first batch just to get it started.
As for whether this is worth it...
Only if you can't obtain NaOH some other way. This will be slow and inefficient and use quite a bit of electricity.
|
|
|
Abdibsz
Harmless

Posts: 8
Registered: 9-6-2019
Location: Mercer Island, WA, USA
Member Is Offline
|
|
Yeah, unfortunately, I can't. And while I was aware that a small amount of Cl+ ions would contaminate my NaOH solution, I thought that the majority of
Cl- ions would end up in the jar with the the anode, while the majority of Na+ ions would end up in the jar with the cathode, for as long as voltage
is applied anyway. Or do you mean that the Cl2 gas could make it to the jar with the cathode and contaminate the NaOH then?
P.s. How do you type with subscripts and superscripts?
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
X[*sub]ee[*/sub]
X[*sup]ee[*/sup]
Without the astricses
Xee
Xee
[Edited on 9-6-2019 by Tsjerk]
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Cl+ ion doesn't
exist in aqueous environment.
|
|
|
Abdibsz
Harmless

Posts: 8
Registered: 9-6-2019
Location: Mercer Island, WA, USA
Member Is Offline
|
|
Oops, sorry, typo. I meant to say Cl-. Wow, that's embarrassing...
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
your setup using a salt bridge is inneficient and is mostly used for lab or theoretical usage
if you want to produce NaOH in a reasonable quantity at reasonable efficiency you need a cuboid cell divided by ceramic porous barriers and each
alternating compartment would be a different polarity.
the voltage through your cell would depend on your desired current running through it and its best you keep it at 5v but the more resistance the
higher voltage you may have to run the cells.
then you need some way of removing the NaOH produced by the cathode compartment while its recycled with fresh NaCl to prevent it from reaching
equilibrium which may cause hypochlorite to start forming in the anode compartment due to the raising of the PH on the anode side.
electrodes used for cathode side would best be stainless steel and on anode side would best be PbO2, Pt, Pb, MMO graphite
MMO requires ph control to keep it at or above 7 or else it may errode in the acidic conditions of the anode compartment this is not a chlorate cell!!
Pb will have high resistance however it produces a coating of PbO2 on the surface but in its alpha form it will flake off after prolonged use and
increase cell resistance.
Pt is good but will erode eventually.
PbO2 on Ti is the best material or PbO2 on graphite
graphite will intercalate
|
|
|
Abdibsz
Harmless

Posts: 8
Registered: 9-6-2019
Location: Mercer Island, WA, USA
Member Is Offline
|
|
A cuboid cell with ceramic porous barriers and alternating compartments with different polarity? I'm not too sure what that would look like. I tried
making a diagram, is this more or less what you mean by that?
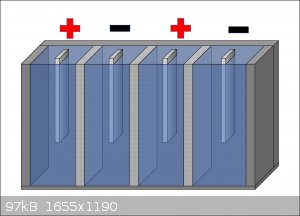
About what you said for the cathode, is stainless steel safe to use? It definitely sounds like it'll be less hassle than mechanical pencil lead, but I
heard that using stainless steel as an electrode was dangerous.
As for anode, would magnetite make a decent electrode? I'm not sure where I could get PbO2, Pt, Pb, MMO, or graphite electrodes without losing a fair
bit of money, but I think I can make some magnetite-coated electrodes easily enough.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
How far from civilization is Mercer Island that you can't go to a hardware store and buy lye?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Abdibsz
Harmless

Posts: 8
Registered: 9-6-2019
Location: Mercer Island, WA, USA
Member Is Offline
|
|
Ahaha, it's an island directly east of Seattle so not that far, but it can be a bit inconvenient if you're a high school student without a
car. Especially since the city recently cut all but one of the bus routes out to alleviate their budget problems...
There is one small hardware store on the north end of the island, but the employees there have started looking at me suspiciously. A few
weeks ago, one even asked if I was planning anything illegal after I bought some copper pipes, moss killer, epsom salts, and batteries...
Plus, my usual excuse that it's for a school project isn't going to work anymore, seeing as school is almost out.
[Edited on 6/11/2019 by Abdibsz]
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
those are all otc stuff, what you do with them regardless of legality is none of their fucking business
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
You could always buy from Amazon.
I don't think you even need a credit card or Paypal.
You used to be able to set up your account with an Amazon gift card.
You can buy a $50 or $100 gift card at par in a lot of stores, including many grocery chains. Safeway sells them.
They have lye, lye based drain cleaners, and food grade NaOH.
Lots of other stuff too.
They have all sorts of chems. I bought potassium chromate from them once.
They have glassware (Laboy is pretty good and pretty cheap), and the odds and ends like filter paper, ph strips, rubber tubing blah blah blah.
I think electrolytic chemical production is pretty cool, but if you just want a pound of Lye in a hurry, You might want to check them out.
If you've got, or are willing to set up a paypal account you can do Ebay, the prices are lower, and you can get all kinds of stuff.
EDIT: Don't know if you can set up a Paypal account if you're under 18, but you can still probably set up an Amazon Acct by the gift card route.
[Edited on 11-6-2019 by SWIM]
|
|
|
Abdibsz
Harmless

Posts: 8
Registered: 9-6-2019
Location: Mercer Island, WA, USA
Member Is Offline
|
|
Yeah, all I was planning on doing with them was make some copper, zinc, and magnesium salts, and electrowinning some zinc if I had enough enough zinc
sulfate left over, but from his questions, I'm pretty sure he thought I was planning on making drugs.
Still, Mercer Island is a pretty small city with a decently active rumour mill and I'd rather not hear any about a suspicious high school student
buying chemicals to do who knows what.
|
|
|
Abdibsz
Harmless

Posts: 8
Registered: 9-6-2019
Location: Mercer Island, WA, USA
Member Is Offline
|
|
Also, about the Amazon idea, my family does have amazon prime, but while my parents have given me permission to do chemistry experiments and such in
the backyard and garage, that's was only because they initially thought I was just planning on doing some elementary school stuff like mixing baking
soda and vinegar or dropping mentos in coke. To be frank, my jars of various coloured solutions, electrolytic cells, electric frying pan sand bath,
and notebook full of equations and calculations are kinda freaking them out. 
So yeah, they turned down that idea when I asked.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by Abdibsz  | A cuboid cell with ceramic porous barriers and alternating compartments with different polarity? I'm not too sure what that would look like. I tried
making a diagram, is this more or less what you mean by that?
About what you said for the cathode, is stainless steel safe to use? It definitely sounds like it'll be less hassle than mechanical pencil lead, but I
heard that using stainless steel as an electrode was dangerous.
As for anode, would magnetite make a decent electrode? I'm not sure where I could get PbO2, Pt, Pb, MMO, or graphite electrodes without losing a fair
bit of money, but I think I can make some magnetite-coated electrodes easily enough. |
stainless steel cathode is fine but not as an anode
magnetite has too much resistance unless its a magnetite coating and i doubt it will survive the chlorine attack.
however i know something that will work which ive done also as an anode but its sacrificial and will drop the efficiency of the cell but it will
produce quite strong lye on the cathode side.
the usage of scrap steel rebar as an anode will generate FeCl2 and FeCl3 in the anode compartment however in the initial run i suggest you add a bit
of HCl to the anode side or even a weak organic acid like citric acid or acetic acid will work just to lower the ph.
I dont know if you will find a use for FeCl3 but hey its a PCB etchant.
This is all because i was trying to create FeCl3 for someone and this method also created NaOH.
also if you are gonna use Pb ensure you have some sodium sulfate or sulfuric acid in the anode compartment to stabalize it
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
These cells will take some time to produce lye depending on the current you run through them.
you will spend less money just buying the lye because the lye you made will have its cost on your electricity bill, cost of cell and electrodes.
assuming you used scrap steel for the anodes well that will be messy and you cant just dump the waste material down the drain.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by Abdibsz  | Hey, this is my first post so I'm not sure if this in the correct section. Anyways, I'm trying to produce NaOH through the electrolysis of salt water.
I'm pretty sure it'd be easier for me to just buy some but that's not an option for me right now. I've attached a diagram of my setup.
The thing is, it's not working. I think it's because of how terrible a conductor distilled water is. What can I add to the water to make it conductive
without drastically lowering the purity of the resulting NaOH solution by too much?
P.s. Sorry for the crap diagram, as you can tell, art isn't my strong suit Lol |
I also wonder what your 5 volt power supply is
if its an adaptor and you plan to make several pounds of NaOH then it will take weeks.
I recommend a 12v old ATX power supply from an old computer and even the 5v rail from it maybe useful for your cell if 12v is too much wastage for
you.
ive made several liters of fairly concentrated NaOH and FeCl3 using that setup in a matter of 3 days.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
If you're in the Seattle area, there's a hardware store in West Seattle that has exactly what you're looking for (Rooto NaOH prills and 93% sulfuric
acid drain cleaner, both fairly cheap). Can't remember the name off the top of my head, but I know it's very close to the farmers' market. One bottle
of each should last you a while, so if you're willing to make the trip every once in a while, it could work out.
That said, I'd be remiss if I didn't encourage some good old-fashioned electrolysis experimentation. If you could find a decent carrier for NaOH in
the salt bridge (paper will fall apart), you could add a small amount of NaOH to the catholyte as well and you should get decent conductivity without
having to worry about chloride contamination. Maybe a clean, non-dyed, wettable fabric would hold up better?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|