Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
SO2 Production
This is possible to produce SO2 by this plan?
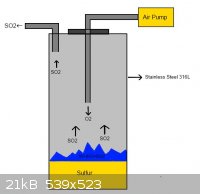
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Yes why not?
BTW you will have to deal with sublimed Sulphur - lots and lots of it - in this scheme.
gsd
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I want to use this plan for making Hydroxylamine.HCl
In this plan i use air( NOT Pure oxygen) and i should control flow rate of air by air pump(high rate may lead to SO3 and low rate cause flame off)
Air pump will be aquarium pump(it is simple  ) )
Stainless steel 316L is resistance to SO2 but i think i should use better alloy
http://www.engineeringtoolbox.com/metal-corrosion-resistance...
Yes i have lot of sulfur in this plan(this is my mistake in drawing  ) )
There is better plan for produce SO2?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
The burning sulfur will constantly tend to go out in this scheme unless the air stream is quite fast and the liquid sulfur is absorbed onto a porous
refractory substance to provide a large surface area for burning.
I have observed that pieces of kaowool (ceramic fibre) impregnated with sulfur and set alight are almost impossible to extinguish by blowing out, and
will burn (the blue flame is only barely visible in daylight, so watch out) for quite a long time. This observation could come in useful here.
Another problem is that the SO2 will always be mixed with an excess of air when it is generated by burning sulfur. This is a big problem when it is to
be used for aqueous chemistry- in the industrial manufacture of Na and K metabisulfite, the oxygen oxidises almost half of the formed bisulfite to
sulfate. The salts have to be separated by fractional crystallisation.
This process is only economically viable due to the cheapness of sulfur, and when a market for the sulfate byproduct exists.
For generating SO2 at home you should buy some K or Na metabisulfite and add HCl to that in a gas generator. The SO2 so obtained is pure and only has
to be dried when it is required anhydrous.
The reaction of aqueous HCl with metabisulfite is very endothermic and the gas generating flask gets very cold (far below 0°C)- so cold actually that
a large amount of the SO2 stays dissolved in the aqueous chloride solution. The flask should be immersed in a warm water bath and either shaken
manually or magnetically stirred to liberate all of the SO2.
[Edited on 13-1-2011 by garage chemist]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Thanks garage chemist
I will have problem If i use smaller amount of sulfur in this plan ?
The reaction of aqueous HCl with metabisulfite is very endothermic???!!!
Wiki wrote sulfuric acid not hydrogen chloride
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
If you drip conc. sulfuric acid into a slurry of bisulfite in water it may be exothermic enough not to get the flask covered with frost like with HCl.
But I would not trust that information from wikipedia unless you have verified it yourself.
I use HCl rather than H2SO4 because it is cheaper and easier to get. It's not that much of a problem to provide a means of heating for the gas
generator flask.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
It does matter which acid you use. The reaction with sulphuric acid will be exothermic as dilution with the water formed in the reaction will
generate a lot of heat.
If you use concentrated sulphuric acid, quite dry sulphur dioxide will pour off.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
I was thinking of the same basic arrangement as at the top of the post as primary sulfur evaporator using a small jet of pure oxygen, then taking the
output gasses into a burner in pure oxygen. Might work as a source for SO3 production using V2O5 catalyst. It effectively would be a flame of S, SO2
burning in an atmosphere of SO2, O2.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by garage chemist  |
Another problem is that the SO2 will always be mixed with an excess of air when it is generated by burning sulfur. This is a big problem when it is to
be used for aqueous chemistry- in the industrial manufacture of Na and K metabisulfite, the oxygen oxidises almost half of the formed bisulfite to
sulfate. The salts have to be separated by fractional crystallisation.
[Edited on 13-1-2011 by garage chemist] |
I belive amount of oxygen (mixed with sulfur dioxide) will decrease if i control the flow rate of air by air pump,but this is difficult to remove all
of it.
Also SO2 can be separate if i do fraction distil at -10c.
|
|
|
Texium
|
Thread Moved
19-11-2023 at 16:22 |