Dr.Bob
International Hazard
    
Posts: 2900
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Better synthesis route to Favipiravir anyone?
The drug Favipiravir is reported to be useful in treating coronacirus, according to public reports, and scientific papers.
https://www.yahoo.com/news/japanese-flu-drug-appears-effecti...
https://www.theguardian.com/world/2020/mar/18/japanese-flu-d...
It may be available in some quantitites, but the routes I find in Scifinder are horrible, and hard to make in simple conditions, or from easy starting
materials. This would be a great target for some brainstorming, however, be aware that if you come up with a great route and publish it here, it
would be come public domain, so you might not get rich, but you would be very poipular with other people and the world. So just a thought.
The structure is linked below, from wikipedia,
https://en.wikipedia.org/wiki/Favipiravir
Here are some reactions below to make it I could find:
I am happy to test out anything that looks promising, if I can get the starting materials. Pyrazines are not common, so a route starting from
commodity chemicals would be great. But I will try to find any SM I can.
Attachment: Favipiravir Syntheses.pdf (1.5MB)
This file has been downloaded 680 times
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2913
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I suspect that this reaction will be extremely difficult regardless of the chosen method of preparation. Adding that fluorine is not easy. For
example, the synthesis of the desfluoro compound is available in one step from aminomalondiamide and glyoxal:
http://wprim.whocc.org.cn/admin/article/articleDetail?WPRIMI...
but it took the researchers a lot more steps -- protections and deprotections -- to achieve the necessary fluorination. The synthesis of this compound
has been a high-value target for a while!
EDIT:
Ullmann reaction of glyoxylamide with 1-fluoro-2-iodoethylene should result in N-fluorovinylation. See:
https://pubs.acs.org/doi/10.1021/ol035355c
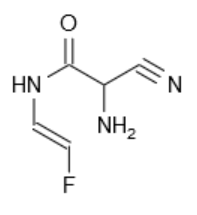
Reaction of this with ammonia and sodium cyanide gives the Strecker aminonitrile -- this is the Strecker amino acid synthesis. See attached picture.
Reaction of the complicated thing with Br2/NEt3 will result in addition to the alkene, Sn2 amine addition and then dehydrobromination to give
3-cyano-5-fluoro-2-pyrazinone.
Selective hydrolysis of this nitrile with H2O2 gives favipiravir.
EDIT2: It may appear that 1,2-bromofluoroethylene would be cheaper. However, bromine monofluoride cannot be isolated. Iodine monofluoride should react
with acetylene at appropriately low temperatures (-78 C) to generate the relevant crosslinker.
[Edited on 19-3-2020 by clearly_not_atara]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2900
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
That's a great example of some ideas. I am curious to look at the des-fluoro, as there are some newer ways to install fluorine coming out now, as
well as the idea of the bottom reaction. I don't expect us to cure a disease, but this will certainly increase demand for favipiravir, at a time
when it may be hard to get from China or India.
So as chemists, this is one of the things we can do to help society. I would ask about making the Gilead drug, remdesivir, but it is one of the more
complex pharma compounds I have ever seen, even in the antivirals, which are commonly tough. So I don;t think it will be easy to scale up without
major facilities. But favipiravir looks much more approachable and also appears to work against many viruses, so maybe more useful. So thanks.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
1.Synthesis of 2-hydroxypyrazine - https://pubs.acs.org/doi/10.1021/ja01126a070
https://patents.google.com/patent/US2805223A/en
2.Duff reaction on 2-hydroxypyrazine-https://en.wikipedia.org/wiki/Duff_reaction
3.P-flourination - https://www.sciencedirect.com/science/article/abs/pii/S00221...
4.Direct conversion of aldehyde to favipiravir - https://www.thieme-connect.com/products/ejournals/pdf/10.105...
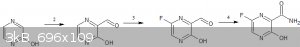
EDIT - Instead of doing a duff reaction in step 2,we can do a Reimer–Tiemann reaction with CCl4 to get the acid directly.Then the last
step would be done with just NH3 and TCT (TCT might not be required if the COOH gets converted to COOF during the flourination  ) )
[Edited on 21-3-2020 by CuReUS]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2913
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
CuReUS: elemental fluorine seems a bit bold for a substrate bearing an aldehyde. Likewise for a substrate bearing an amide. Maybe form a cyclic
acetal? Of course, elemental fluorine always seems bold...
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | | CuReUS: elemental fluorine seems a bit bold for a substrate bearing an aldehyde. Likewise for a substrate bearing an amide. Maybe form a cyclic
acetal? |
Are you suggesting the aldehyde would be turned into a diflouro compound ? In that case,we will be
able to hydrolyse it back during the workup and no protection would be required.
But what happens to amides when they react with flourine ?
I found some papers on the electrochemical fluorination of organic compounds
https://link.springer.com/article/10.1023/B:RUJO.0000036072....
https://pubs.rsc.org/en/content/articlelanding/2011/cc/c1cc1...
| Quote: | | Of course, elemental fluorine always seems bold... |
Desperate times call for desperate measures 
But this paper suggests some ways to tame F2 - https://www.sciencedirect.com/science/article/abs/pii/S00221...
1.Using Flourodec for generating F2
2.Diluting it with N2
3.Using strong protic solvents like H2SO4 or HCOOH
I
wonder if we could p-flourinate that using the paper I linked to get favipiravir ?
[Edited on 22-3-2020 by CuReUS]
|
|
|
Tsjerk
International Hazard
    
Posts: 3040
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Does anyone have a full synthesis of remdesivir? Not because I want to produce it but because i can't find it.. I'm a bit sick. Me and my girlfriend
are quarantined because we scored a 100% symptoms. We are fine though.
|
|
|
cirice1
Harmless

Posts: 12
Registered: 8-1-2019
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | | Does anyone have a full synthesis of remdesivir? Not because I want to produce it but because i can't find it.. I'm a bit sick. Me and my girlfriend
are quarantined because we scored a 100% symptoms. We are fine though. |
https://newdrugapprovals.org/2020/03/12/remdesivir-%e3%83%ac... scroll down a little and you'll find synthesis and patents
|
|
|
Fery
International Hazard
    
Posts: 1099
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Tsjerk if you and your gf are not elderly and do not have other serious diseases you have very good chances that your immunity destroys every
infection faster than you synthesize the antiviral compound
your last sentence is very good sign - "we are fine though"
these special medicals usually require advanced factory/lab equipment
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2913
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
So I thought a bit more about the route I posted.
Unfortunately, glyoxylamide is not commercially available. It also might not be very stable considering the tendency of aldehydes to react with
primary amides -- an equilibrium, but glyoxylates are very reactive aldehydes. And I'm not sure that the N-vinylation conditions will tolerate
aldehydes. However, I had forgotten that they do tolerate amines.
So instead we start with ethyl glyoxylate and sodium cyanide with an excess of ammonia in ethanol. The aminonitrile forms first, and then the ester
undergoes aminolysis. This should give mostly the stable 2-cyanoglycinamide, which can then be N-fluorovinylated with 1,2-iodofluoroethylene.
In the third step I think it would be most advantageous to start with the ammonium salt in some polar solvent (that isn't easily oxidized) and add one
equivalent of bromine, which will add to the alkene, followed by base, which will close the ring. There is the question of whether we will get a
5-membered ring or a 6-membered ring; the former is typically favorable, but the angles around the amide bond are fixed at 120 degrees, which promote
the formation of the correct product. Then adding one more equivalent of bromine should take us to the pyrazine; I'm not sure if the 1-, 3- or 4-
position will react first, but either should react with one equivalent of bromine to give a product that easily eliminates to pyrazine.
Scheme attached. Of course we hope the fluorine atom doesn't eliminate as HF under the reaction conditions. I expect it to be "kinetically protected",
that is, lost relatively slowly, so the reaction can still complete.
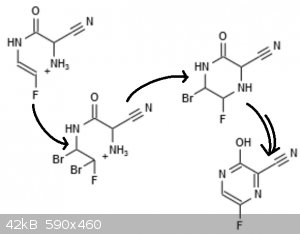
|
|
|