Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Preparation of benzyl cinnamate (a moderate difficulty ester)

52 mL of toluene and 0.5 mL of conc. sulfuric acid were added to a flask with a 20 mL dean-stark trap and a condenser. This mixture was refluxed for a
short time, until no more water collected in the trap, to form para-toluenesulfonic acid in situ.
Fischer esterification.
Once the toluene cooled down, 14.5 mL benzyl alcohol and 22.8 grams cinnamic acid (1.1 mol equiv) were added. Initially the cinnamic acid doesn't
dissolve.

Once heated, the cinnamic acid dissolved into the toluene. It took less than an hour for water to stop collecting in the dean-stark trap, which is
when heating was turned off.

After cooling to room temp, the crude benzyl cinnamate precipitated to form a slush.

About 40 mL of diethyl ether was added to dissolve the solids.

This ether solution was washed with water, sodium bicarbonate (removes cinnamic acid but i'm not sure i washed enough so product may contain some
cinnamic acid), water, and saturated sodium chloride, then dried with anhydrous calcium chloride pellets.

The diethyl ether was recycled by fractional distillation. The toluene was evaporated outdoors with stirring, mild heating, and a fan. Then the
mixture was heated to 230 C to expel any remaining benzyl alcohol.
Crude benzyl cinnamate before distilling:

The crude benzyl cinnamate was distilled twice by vacuum distillation, with a short path condenser and condenser water circulating at 50 C, to receive
a light yellow liquid. My vacuum pump gets about 28 in hg and it boile dat around 250 C. Even after 2 distillations I couldn't get rid of the yellow
color. Theoretically it should be white, but the compound is often reported to be yellow. I hope it's not horrible practice to use a bunsen burner
here, but there were no volatile organics involved in this distillation and I didn't have enough sand at the time to make a sand bath.

This is what the product looked like immediately after 2 vacuum distillations, while still liquid:

At room temperature it is a white soft waxy solid, but I fear that some cinnamic acid contamination is depressing the melting point. I don't smell any
cinnamic acid so it's probably minor.

Tomorrow I will bring it to university lab for melting point determination and GC-MS

[Edited on 11-3-2020 by Cou]
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Cou, very nice !!!
I plane to prepare butylcinnamate / pentylcinnamate as they should have cocoa scent and I love cocoa...
I will very likely start from benzaldehyde and ethyl acetate and prepare ethyl cinnamate and then perform transesterification with 1-butanol / 1-pentanol when ethanol will distill off so the final ester should stay.
I already prepared slightly more than 10 g of cinnamic acid which is too little, ethyl cinnamate could be better.
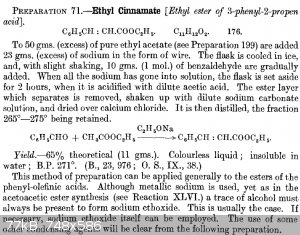
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Cou: Very nice writeup! I really like your ester preparations, it's interesting how many scents you can make from simple reagents.
Fery: Cocoa scent, this sounds nice  . .
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Here is the GC-MS spectrum.

The GC peak at 17.3 minutes is definitely the benzyl cinnamate. It has the prominent 92 m/z mass spectrum peaks that are found in aromatic compounds.
None of the other GC peaks have that large 92 peak. All the other GC peaks have mass spectrum ions that are too big to be either cinnamic acid or
benzyl alcohol. I have no idea what the other ones are, especially that large one at 14.2 minutes.
Does anyone have access to a NIST library so we can find out what those other compounds are?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4279
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I made ethyl cinnamate once, but because I can't do a vacuum distillation, I had a lot of trouble trying to purify it. Methyl cinnamate was much
easier.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Anyone want to run this thru a library to identify the peaks?
https://drive.google.com/file/d/160caMtFI9m75HNBh9vs8v0q6XiC...
edit: unfortunately it seems that the large 14 min peak is cinnamic acid. Ions sometimes can dimerize and sh*T to form ions larger than the original
molecule. My base wash wasn't thorough enough; you have to keep going until absolutely no more CO2 forms. this can be quickly fixed by melting the
product, waashing w/ bicarb, then water, then sat. NaCl, then drying w/ CaCl2, then a final vacuum distillation.
[Edited on 11-5-2020 by Cou]
|
|
|