Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
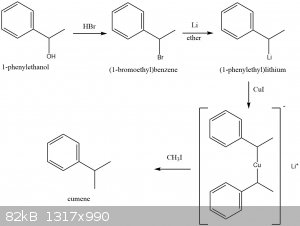
Cumene synthesis idea from 1-phenylethanol (commercially available fragrance compound)
i'd like to try this one day as a project, but i've been so busy with other things that i haven't even had time to do my first home grignard reaction,
let alone get experience with organolithium and formation of gilman reagents. I think organolithiums require a schlenk line.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2660
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
That might work, but I would expect styrene and its polymer to be a major side product, like every other similar reaction I have tried with 2 carbon
side chains on benzene.
If you want cumene, I would just react isopropyl bromide or chloride with benzene + AlCl3 or such. Easy to make those from isoPrOH and HBr/HCl.
Much simpler, cheaper, and only one step. Still might get some mixtures and side products, but simple enough to distill that mess. I'm pretty
sure I got cumene as a side product in some reaction years ago, but can't remember which now. Of course propylene would work also, but harder to
find that OTC.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Boiling point of benzene: 80.1 C
Boiling point of cumene: 152 C
Boiling point of 1,3-diisopropylbenzene: 203 C
Boiling point of 1,3,5-triisopropylbenzene: 232-236 C
Seems like if you use an excess of benzene, it wouldn't be hard to use fractional distillation to separate all the different friedel-crafts alkylation
products.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2660
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I agree that you can distill any reactions to get decent separations, just figure that Lithium and methyl iodide are more precious than isopropanol to
most people. But if you have them, give it a shot.
|
|
|