Hydrogen Peroxide - Illustrated Practical Guide
Hydrogen peroxide is available readily as a disinfectant in pharmacies and grocery stores, but may only be obtained easily in low concentrations often
as 3% or 6% solutions.
Higher concentration peroxide is sold for animal disinfectants, pool/spa treatments, hair bleaching. Some may contain other chemicals, including
stabilizers, so read the label first.
PROPERTIES
Hydrogen peroxide is unstable and slowly decomposes in the presence of light
SOLUBILITY
soluble
Ethanol
Methanol
Ethyl acetate
Ethyl ether
insoluble in petroleum ether(Light petroleum)
Danger Note: working with small quantities is a very good idea when working with potentially energetic materials.
BLEVE explosion is possible with hydrogen peroxide
Boiling liquid expanding vapor explosion
Above roughly 70% concentrations, hydrogen peroxide can give off vapor that can detonate above 70 °C (158 °F) at normal atmospheric pressure. This
can then BLEVE the remaining liquid. Distillation of hydrogen peroxide at normal pressures is thus highly dangerous, and must be avoided
The danger of explosive decomposition during distillation appears to be much greater with solutions which have already been concentrated by extraction
with ether
STABILIZERS
In some applications, a high degree of stabilization is needed; whereas, in others (e.g., drinking water treatment or semiconductor manufacture)
product purity is more important. For most environmental applications, H2O2 stabilization does not affect product performance.
Sodium citrate
Phosphoric acid
Sodium silicate
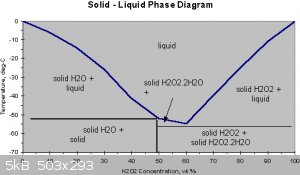
This is how freezing out to concentrate the solution works
Hydrogen peroxide and water form a eutectic mixture, exhibiting freezing-point depression down as low as –56 °C; pure water has a freezing point of
0 °C and pure hydrogen peroxide of −0.43 °C. The boiling point of the same mixtures is also depressed in relation with the mean of both boiling
points (125.1 °C). It occurs at 114 °C. This boiling point is 14 °C greater than that of pure water and 36.2 °C less than that of pure hydrogen
peroxide.
.
Freezing the mixture
"The freezing points of H2O2 and H2O are very similar, so this is tedious, but it is quite effective. Put the solution in the freezer and skim off ice
crystals as soon as they form. The ice crystals, if small, will be nearly pure water. This will increase the H2O2 concentration of the unfrozen
portion."
Heating and evaporation
Dessication
Vacuum distillation
Appearance
Very light blue color; colorless in solution
DENSITY
1.11 g/cm3 (20 °C, 30% (w/w) solution )
1.450 g/cm3 (20 °C, pure)
PEROXIDE COMPOUNDS
Lithium peroxide
Sodium peroxide
----
Potassium peroxide (atomistry.com)
Several peroxides have been described. The tetroxide, K2O4, or K2O2,O2, is produced by burning the metal in air or oxygen, de Forcrand heats the
potassium first in a current of nitrogen, then in air, and finally in pure oxygen at a temperature between 180° and 200° C. The product is a
sulphur-yellow, very hygroscopic powder, its heat of formation from the elements being 137.74 Cal. It is decomposed by water, with evolution of oxygen
and formation of potassium hydroxide and hydrogen peroxide:
K2O4 + 2H2O = 2KOH + H2O2 + O2.
With dilute sulphuric acid it yields hydrogen peroxide.
When the tetroxide is heated at 480° C. under a pressure of 1 mm. of mercury, it is converted into potassium trioxide, K2O3, a yellow, crystalline
substance, from which dilute sulphuric acid generates hydrogen peroxide. Its heat of formation from the elements is 124.34 Cal.
At -50° C. oxygen reacts with a solution of potassium in liquid ammonia to form the tetroxide and trioxide, and also potassium dioxide, K2O2. The
heat of formation of the dioxide from its elements is about 108.8 Cal.
Potassium hydrogen tartrate and potassium fluosilicate
are sparingly soluble substances, so that concentrated aqueous solutions of hydrogen peroxide may be prepared by treating potassium peroxide with
concentrated solutions of tartaric acid or hydrofluosilicic acid. Barium peroxide, however, is the substance commonly employed.
-------------
Sodium Percarbonate.
Sodium percarbonate can react with sulfuric acid to form sodium sulfate which can be separated out by chilling the solution and Hydrogen Peroxide
Or hydrogen peroxide can be reached out with a solvent.
Sodium percarbonate is an abduct of sodium carbonate and hydrogen peroxide. sodium carbonate is insoluble in alcohol but hydrogen peroxide is soluble
in alcohol couldn't anhydrous ethanol or isopropanol be used due to isopropanol properties. It can only oxidize alcohol with a catalyst btw. So it
would be
Sodium carbonate perciptate with hydrogen peroxide/isopropanol
I do like the idea of hydrochloric acid and sodium percarbonate due to sodium chloride being insoluble in hydrochloric acid also hydrogen chloride gas
could be used to reduce the amount of water used
Also for isopropanol hydrogen chloride is soluble and sodium chloride is not even without hydrochloric acid.
Potassium peroxide is treated with a solution of tartric acid
Which forms insoluble potassium tartrate and hydrogen peroxide of a concentration depending on how much water is used for the reaction
Barium peroxide (atomistry.com)
Some of the earlier processes depending on the use of barium peroxide were inconveniently cumbrous. Thus Thenard, early in the nineteenth century,
recommended a method of which the following description is merely an outline, many details being omitted. Powdered barium peroxide was dissolved in
dilute hydrochloric acid and the barium then precipitated by the careful addition of sulphuric acid. The resulting solution, containing hydrogen
peroxide and hydrochloric acid, together with impurities from the barium peroxide and probably a little sulphuric acid, was treated with a little
barium hydroxide solution or barium peroxide emulsion when any heavy metals were precipitated at the same time as the sulphuric acid; by the artifice
of previously introducing a little phosphoric acid, any manganese or iron could be simultaneously removed as their respective phosphates, whereas, if
allowed to remain, they would have caused rapid decomposition of the hydrogen peroxide. After this treatment the solution contained only hydrogen
peroxide and barium chloride, the latter of which was removed by conversion with silver sulphate into silver chloride and barium sulphate. The clear
solution of hydrogen peroxide thus obtained possessed a high degree of purity and was finally concentrated in a vacuum over sulphuric acid.
The disadvantage attaching to the direct conversion of barium peroxide into an insoluble salt by treatment with such an acid as sulphuric or carbonic
acid, lies in the sparing solubility of the first-named substance which causes the reaction to be incomplete. This difficulty can be remedied by
previous prolonged agitation of the anhydrous barium peroxide with water, by which treatment it becomes converted into the hydrated and more reactive
compound BaO2.8H2O. This readily yields dilute solutions of hydrogen peroxide when treated with aqueous sulphuric, hydrofluoric, hydrofluosilicic,
phosphoric, or carbonic acid. In the case of the last-named acid, it is important to use an excess.
Exceptionally pure hydrogen peroxide was obtained by Maass and Hatcher from a 3 per cent, solution prepared in the usual way from commercial barium
hydroxide. This was first concentrated to 30 per cent., using a special type of sulphuric acid concentrator, but this product required very careful
handling as all the non-volatile impurities had also been concentrated in the process. The liquid was now distilled at 65° C. under a pressure of 10
mm. of mercury maintained with a sulphuric acid pump. Qualitative analysis of the distillate showed the absence of all non-volatile impurities;
sulphuric acid was also absent, but in those cases where the original peroxide solution contained large amounts of chloride, some hydrochloric acid
was present in the distillate unless the original solution was first made alkaline. The distillate was now exceedingly pure and contained on the
average 85 per cent, of peroxide, the remainder being water. This was concentrated to 90 per cent, peroxide over sulphuric acid in the special
concentrator referred to above. It now remained to remove, in so far as possible, the remaining 10 per cent, of water. This was effected by systematic
fractional crystallisation, a product containing 99.93 per cent. of peroxide being ultimately obtained. Concentration of hydrogen peroxide in the
commercial solution containing about 30 per cent.
H2O2 is known as perhydrol.
-------------
Calcium peroxide
Strontium peroxide
Urea peroxide
one method involves using calcium or barium hydroxides to precipitate peroxide, leaving urea dissolved, and, after filtering out the alkaline earth
metal peroxide, recovering hydrogen peroxide from it with sulfuric acid. This, however, requires a very concentrated solution of the adduct, decanted
or vacuum-filtered from a sludge of wet powdered percarbamide.
Synthysis of urea peroxide
https://youtu.be/qoqMHbxN4i8
Hypothesis of other reactions
Urea peroxide
I'm not sure if urea is soluble in ethyl acetate
But this could be an interesting path to Hydrogen peroxide.
Cymene peroxide
Serine peroxide
Hydrogen peroxide is one of the most versatile oxidation reagents, still it has not fully been exploited by synthetic chemists since anhydrous (let
alone pure) hydrogen peroxide requires hazardous preparation protocols. We have recently reported on the crystallization of serine and other amino
acid perhydrates, thus paving the way for a new method for laboratory-scale production of anhydrous hydrogen peroxide solutions. Serine is insoluble
in most organic solvents (e.g., methanol, ethyl acetate, and methyl acetate) that readily dissolve hydrogen peroxide. Moreover, since the adduct of
hydrogen peroxide and serine is unstable in these organic solvents, crystalline serine perhydrate readily decomposes to give anhydrous solutions of
hydrogen peroxide and crystalline precipitate of the amino acid. This procedure can then yield an anhydrous hydrogen peroxide solution in a single
step. Moreover, filtration of the amino acid, and room temperature evaporation of the volatile solvent (e.g., methyl acetate), yields over 99%
hydrogen peroxide.

Update about serine peroxide
Observation in light the crystal appears to "melt" the water around it contains suspended bubbles
Is that it is sensitive to light and slowly decomposed to oxygen an water and the adduct slowly breaks down. Leaving water and serine
Using Cymene
another method 80-90% hydrogen peroxide starting material can also be obtained by mixing 30% hydrogen peroxide solution with twice its amount of
p-cymene, followed by distillation of the mixture at 50°C, using an aspirator vacuum. Most of the water and p-cymene is thus removed. After
mechanical separation of the remaining p-cymene-H2O2 mixture.
Sodium perborate
Sodium persulfate
Sodium perphosphate
The addition of H2O2 at low temperatures.
Anodic oxidation.
Hydrogen peroxide does not combine with orthophosphoric acid. But when metaphosphoric acid or phosphorus pentoxide were treated with 30 per cent,
hydrogen peroxide at 0° C., solutions were obtained which had oxidising properties and which by analysis proved to contain per mono phosphoric acid,
H3PO5. With pyrophosphoric acid in excess, perdiphosphoric acid, H4P2O8, was obtained.
Slightly acid or alkaline phosphate solutions of the alkali metals, etc., combine with varying proportions of H2O2 in a loose manner. Such solutions
give the reactions of H2O2.
Electrolytic oxidation. The perphosphates of the alkali metals and of ammonium have been prepared in this way. As in the electrolytic production of
persulphates low temperatures are advantageous and the presence of fluorides and chromates increases the yield, probably by maintaining a high anodic
over-voltage. A low anodic current-density (e.g. about 0.015 amp./cm2.) is favourable. The electrolyte may consist of a solution of KH2PO4 with
fluoride and chromate. On evaporation at 100° C. after the electrolysis potassium perphosphate, K4P2O8, can be crystallised.
Perphosphates of ammonium may be prepared in good yields by electrolysis, but auto-oxidation and -reduction may occur with the production of ammonium
phosphate and ammonium nitrate.
Perphosphates of rubidium and caesium are more easily prepared, even in the absence of fluorides or chromates. The permonophosphates of these metals
however require in their preparation rather higher current-densities.
Potassium perphosphate gives with silver nitrate a dark precipitate which changes to white Ag3PO5, then to yellow Ag3PO4 with evolution of ozone and
oxygen.
--------------------------------------------------
Perhaps of only theoretical interest, very 'High Test Hydrogen Peroxide' can be formed, at least in small amounts, to quote a source (see 'The gas
phase reaction of singlet dioxygen with water: A water-catalyzed mechanism' by Xin Xu, Richard P. Muller, and William A. Goddard III, in PNAS March
19, 2002 99 (6) 3376-3381; https://doi.org/10.1073/pnas.052710099:, link: http://www.pnas.org/content/99/6/3376):
"Recently, Wentworth et al. (1, 2) from the Scripps Research Institute reported the surprising result that Abs, regardless of source or antigenic
specificity, have an ability to catalyze the generation of H2O2 in a highly efficient manner and by a mechanism that involves the oxidation of H2O by
singlet oxygen molecules, O2 (1Δg). ..... To understand how Abs can carry out this remarkable and unexpected chemistry, we need to understand how 1O2
can interact with H2O to produce H2O2. Preliminary mechanistic investigation (2)§ suggested that 1O2 might be able to convert H2O to H2O2 via the
formation of hydrogen polyoxides, H2O3 and H2O4."
where one might call H2O3 and H2O4 high test grades of hydrogen polyoxides.
[On addition of 30 per cent, peroxide to sodium hydroxide or ethoxide a precipitate of sodium perhydroxide is obtained to which the formula
2NaHO2.H2O2 is given. This possesses marked basic properties, and on saturation with carbon dioxide yields the acid percarbonate, NaHCO4. The same
compound is formed when hydrogen peroxide is added to sodyl hydroxide, NaO.OH. This latter substance was first prepared by Tafel by the action of
sodium peroxide upon well-cooled absolute alcohol.
With potassium hydroxide two compounds have been obtained, namely, 2KHO2.H2O2 and 2KHO2.3H2O2.
On passing dry ammonia into a solution of pure hydrogen peroxide in absolute ether at -10° C., or into pure anhydrous peroxide at 0° C., ammonium
perhydroxide, NH4O.OH, or NH3.H2O2, crystallises out. Further addition of ammonia is stated to convert this into an oily mass which solidifies at
about -40° C. and has the composition (NH4)2O2, and is therefore ammonium peroxide, the analogue of sodium peroxide. The crystals of ammonium
perhydroxide melt at 24.5° C. to an oily liquid, which is stable in the entire absence of water.
Reactive chem
https://www.bitchute.com/video/NbX4SWk9a1hA/
Concentrating 3% hydrogen peroxide by
Dessication with calcium chloride
Distillation under reduced pressure with sulfuric acid
Synthesis of hydrogen peroxide
https://youtu.be/LB2pO7OteDU
Sodium peroxide
https://youtu.be/ZqEWUw6sgpA
Potassium peroxide
Several peroxides have been described. The tetroxide, K2O4, or K2O2,O2, is produced by burning the metal in air or oxygen, de Forcrand heats the
potassium first in a current of nitrogen, then in air, and finally in pure oxygen at a temperature between 180° and 200° C. The product is a
sulphur-yellow, very hygroscopic powder, its heat of formation from the elements being 137.74 Cal. It is decomposed by water, with evolution of oxygen
and formation of potassium hydroxide and hydrogen peroxide:
Sodium percarbonate
https://youtu.be/yHATAwpfSTU
Sodium borate added to glacial acetic acid will yield peracetic acid, which can be used to oxidize aromatic amines to nitro groups,
Sodium perborate & barium peroxide
https://youtu.be/THuBQV96RKM
Sodium perborate 1
https://youtu.be/DtKRwcweVow
Sodium perborate2
https://youtu.be/cJ2DxN5Ly0Y
Properties of high test Hydrogen peroxide
https://youtu.be/hJSWTPOa4T8
Concentrating and measuring
https://youtu.be/nVAe__ToAOY
http://antenna-theory.com/high-test-peroxide.php
-------------------
Threads
Hydrogen Peroxide from Sodium Percarbonate
http://www.sciencemadness.org/talk/viewthread.php?tid=155546
[Edited on 29-11-2020 by symboom]
http://www.sciencemadness.org/talk/viewthread.php?tid=15881
http://www.sciencemadness.org/talk/viewthread.php?tid=16726
http://www.sciencemadness.org/talk/viewthread.php?tid=126644
[Edited on 30-11-2020 by symboom]
[Edited on 3-12-2020 by symboom]

|