SplendidAcylation
Hazard to Others
  
Posts: 196
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Is trichloroisocyanuric acid actually an acid?
Wikipedia (famous for its trustworthiness) states:
"Salts of trichloroisocyanuric acid are known as trichloroisocyanurates."
At first glance, this makes sense; It's an acid, surely it forms salts, as does DCCA...
However, if we look at the structure of cyanuric acid:

We see that it exists as a tautomer between the enol (left) and keto (right) forms;
The acidity of cyanuric acid is due to the phenolic character of the hydroxy groups, which are only present in the enol tautomer, so you could say
only the enol form is actually an acid.
The enol and keto forms exist in equilibrium.
I'd imagine that, were one to neutralize cyanuric acid with a base, one would be removing the enol tautomer, thus, as Le Chatelier's principle states,
the equilibrium will counteract this change by making more of the enol tautomer, until eventually you have cyanurate ions and none of the keto form
left...
This would work for DCCA as well:
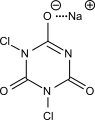
But as for TCCA, it doesn't have any hydrogen atoms in the molecule at all, therefore, quite straightforwardly, it can't be a Brønsted acid, as it
doesn't have any protons to donate.
It's still possible for it to behave as a Lewis acid, perhaps a resonance structure could be drawn where one of the carbons would be electron
deficient, and a Lewis adduct could form, perhaps it has Lewis acidity like boric acid; I'm not sure...
Still, such a Lewis adduct would not be analogous to the cyanurate or the dichloroisocyanurate ion...
Perhaps the best evidence that trichloroisocyanurates don't exist is the complete lack of any actual examples of such compounds?
Another thing, just because it has "acid" in the name, doesn't necessarily (as far as I know) mean that it is an acid; In this case it might simply
mean that it is the trichloro derivative of a compound that actually is an acid?
Anyway, forgive me if any of my conclusions are erroneous, although I absolutely love every aspect of chemistry, I didn't even know what a Lewis acid
was a year ago, I'm very much still learning 
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
No TCCA is not an acid, it is just in the name as you say.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Just want to chime in on this, Cyanuric acid is not much acid like either
Dropping powdered Cyanuric acid in a cup of sodium hydroxide does
nothing. Heating with sodium carbonate takes hundreds of degrees Celsius
and several hours to only partially react. As far as being an acid
Its a really crappy one.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
draculic acid69 - as I reported in the past cyanuric acid does react with some metal carbonates and even with fine copper powder, although the latter
took near 100 hours of stirring hot before yielding a lovely dark purple compound. So in this sense I thought it did behave acid-like, with my limited
understanding.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
draculic acid, did you try adding a solution of cyanuric acid to a solution of NaOH?
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Every compound, capable of splitting off a H(+)-entity, can be considered an acid (a Brønsted acid), even if it does so only relucantly. E.g., H3BO3
(boric acid) practically is not dissociated in H(+) and H2BO3(-), but if a strong base is added, then this reaction does occur to a larger extent.
Some acids are so weak that in water they hardly are capable of splitting off H(+), but in other solvents, or when crystallizing, they can. An example
is the extremely weak acid HS(-). In water, this does not split to S(2-) and H(+). It actually is the reverse. If Na2S is dissolved, then you get a
solution with HS(-) ions and OH(-) ions. But when a strongly alkaline solution of Na2S is allowed to vaporize, then crystals of Na2S.9H2O are formed,
and then the extremely weak acid still acts as acid.
This is also how it works with cyanuric acid. It is extremely weak, but it can act as acid. It is not a 'crappy' acid, it simply is a very weak acid.
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 196
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Thanks for the replies!
The pKa of cyanuric acid seems to be about 7 according to this:
https://pubs.acs.org/doi/pdf/10.1021/jp9053583
Since the pKa of water is 14 or thereabouts, one would imagine that the equilibrium would lie quite a bit to the right...
Cyanuric acid + OH- <--> Cyanurate- + H2O
@draculic acid69 perhaps the strong heating is required to drive off water and push the equilibrium further to the right?
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Can't remember.trying to get any amount of Cyanuric acid to dissolve
In a cup was pointless. Unless you want to use a few literature of water
a solution isn't Gunna happen.the stuff is very insoluble
[Edited on 23-3-2022 by draculic acid69]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by draculic acid69  |
Can't remember.trying to get any amount of Cyanuric acid to dissolve
In a cup was pointless. Unless you want to use a few literature of water
a solution isn't Gunna happen.the stuff is very insoluble
[Edited on 23-3-2022 by draculic acid69] |
This is exactly the reason I asked. The reason for the acid to be "crappy" is not the fact it is a weak acid, it just doesn't dissolve. It would be
interesting to see how fast it reacts in solution as it would say something about the equilibrium between the tautomers, but I suspect that
equilibrium to be quickly reached.
[Edited on 23-3-2022 by Tsjerk]
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Quote: Originally posted by draculic acid69  |
Can't remember.trying to get any amount of Cyanuric acid to dissolve
In a cup was pointless. Unless you want to use a few literature of water
a solution isn't Gunna happen.the stuff is very insoluble
[Edited on 23-3-2022 by draculic acid69] |
The solubility of cyanuric acid in water is much better near / at water boiling point. 5g acid will dissolve in 200ml boiling water giving a
completely clear solution.
|
|
|