aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Selective Reduction of a cyclic di-imine species
Recently, I synthesized 2,4-Diphenyl-7,8-dimethyl-3H-1,5-benzodiazepine from Benzil and
4,5-dimethylphenylene-1,2-diamine. I was able to get around 1.0 gram (58% yield) from the synthesis and wanted to try some nitrogen
chemistry with it.
My idea was to selectively, or methodically, reduce one of the two imines. However, due to the almost entirely equal nature of the imines, they both
should be reduced via any of the main go-to's for reduction. My idea then was to try and reduce both nitrogens and then slectively protect one and
then oxidize the other.
The problem i see here is that unless I can speedily acetylate one of the two nitrogens, i am bound to have a mixture of products.
Does anyone have any good literature on this, or at least maybe some words of wisdom regarding this?
PS: Something deep inside tells me this is a job for testing The borohydrides (cyano, triacetoxy, etc.), but I am not confident in my judgement.
 
The pictures above show the crashed out product from the synthesis. Melting point was as expected for literature.
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
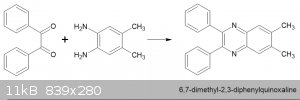
Hmmm. I thought diazepines were 7 member rings, if you condense benzil with and 1,2-phenylenediamine won't you just get a six member quinoxaline. In
your case you should get 2,3-diphenyl-6,7-dimethylquinoxaline! ad the heterocyclic nitrogens are identical since the molecue is symmetrical. Like
this:-
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Although your point should be correct,
Quote: Originally posted by Boffis  | Hmmm. I thought diazepines were 7 member rings, if you condense benzil with and 1,2-phenylenediamine won't you just get a six member quinoxaline. In
your case you should get 2,3-diphenyl-6,7-dimethylquinoxaline! ad the heterocyclic nitrogens are identical since the molecue is symmetrical. Like
this:-
|
A paper published in 2014/2015 by Samigullina et al. regarding benzodiazepines shows the exact synthesis shown above, run in glacial acetic acid,
yields the 7 membered diazepine ring.
Citation:
| Quote: |
Samigullina, A.I., Gubaidullin, A.T., Mustakimova, L.V. et al. Influence of the nature of the substituent on the supramolecular synthon in crystals of
benzo[1,4]diazepine derivatives. Russ Chem Bull 63, 1444–1450 (2014). https://doi.org/10.1007/s11172-014-0617-4
|
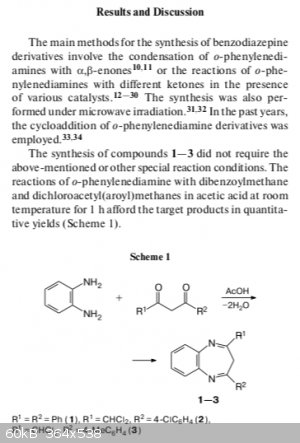
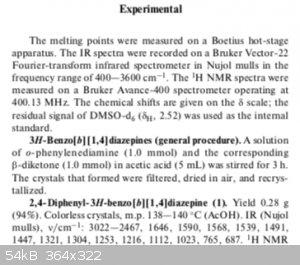
After running this at a larger scale, I was able to yield 58% (0.9909g) of the product. The crystals were ever so slightly tinged yellow/orange, but
the crystals melt very close the literature value (found: 146-148C, lit: ~152C not shown in aforementioned paper). Below is some pictures of the
actual crystals, which look different than they should for a 6-membered ring closure. I would expect some deeper coloration for the structure you
proposed, or at least that's what my colleagues suggest.
  
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
CORRECTION
Welp, I realized I said benzil, my appologies. Didn't catch that.
Further correction, just got my NMR back....
Wouldn't you know, I used acetylacetonate on accident. Probably an error fueled by my lack of sleep, slave wages at the uni, and 3 open projects on 3
entirely different fronts.
[Edited on 4-11-2023 by aab18011]
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
|