DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Catalytic hydrogenation of methyl 3,4,5-trimethoxycinnamate using Cu/Co salts and sodium borohydride
After my experiments with the synthesis of 3,4,5-trimethoxycinnamic acid and methyl 3,4,5-trimethoxycinnamate, I have been looking into ways of
reducing the double bond.
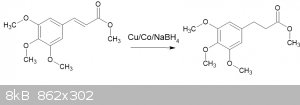
There is a wide body of literature out there on using Pd/C, hydrogen, pressure etc, but I've wanted to avoid the precious metals and generating
hydrogen.
Nickel and cobalt salts, in conjunction with sodium borohydride, also has many papers written about it, but I've not had a great deal of consistent
success with any until I found this paper:
"Inexpensive and rapid hydrogenation catalyst from CuSO4/CoCl2 — Chemoselective reduction of alkenes and alkynes in the presence of benzyl
protecting groups" (Ficker, Svenningsen, Larribeau, Christensen, 2018; doi 10.1016/j.tetlet.2018.02.026)
This paper describes a very straightforward reduction technique for alkenes. One of the trial reductions in the paper was methyl cinnamate; I simply
used the same ratios as used there.
Given the small ratio of cobalt salts used, I decided to make up a stock solution to last me several experiments.
0.39g CoCl2.6H20 (1.64mmol), plus 4.09g CuSO4.6H20 (16.4mmol) was dissolved in 80ml of MeOH, yielding a solution with a 0.2M concentration of Cu
salts.
Experiment
5.22g of methyl 3,4,5-trimethoxycinnamate (20.67mmol) was added to 50ml of MeOH. To this was added a 3.1ml aliquot of the stock solution prepared
earlier, giving a 3 mol% Cu and 0.3 mol% Co catalyst loading.
The solution was then gently heated to approx 45C until all the ester dissolved. Heat was then turned off.
2 equiv of NaBH4 (1.56g) was then added in small portions over a period of 20 - 25 minutes.
A black precipitate forms immediately on the addition of the first portion, and copious gas is evolved. I waited until most of the gas evolution
ceased before adding the next portion. The temperature rose slightly during the addition of the NaBH4 but did not exceed ~ 48C over the duration of
the experiment.
Once the reaction is complete, work up is straightforward. I tried a couple of different techniques to remove the black precipitate, but it is so fine
that it goes through most filter papers. Use of diatomaceous earth helped somewhat. In the end, simply adding water and extracting with CH2Cl2 worked
well as the black precipitate remained in the aqueous layer.
The DCM extracted organic layer was dried with MgSO4, and solvent evaporated. The raw product, a thick oil at this stage, will gradually solidify. For
purification, recrystalize from a 2:1 MeOH:water mixture and then refrigerate - it takes several hours for the final product to come completely out of
solution.
I ended up with a crystalline material "methyl 3-(3,4,5-trimethoxyphenyl)propanoate" with a MP of 43-44C (I could not find a literature reference for
a MP), and using a 1:3 EtOAc:Hexane eluents, obtained an Rf of 0.41. In comparison, the source material MP is 99-100C, with an Rf of 0.43.
My yield was startlingly good - 4.5g, equating to 17.7mmol, or 85%.
It's nice to have wins occasionally...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Very nice!
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
sussyn
Harmless

Posts: 7
Registered: 14-8-2015
Member Is Offline
Mood: No Mood
|
|
Indeed, very nice.
Magnesium in methanol will do this reduction. I'd be interested in a comparison.
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Hi sussyn,
Prompted by your comment, I went looking through the literature and found "Magnesium-methanol as a simple convenient reducing agent for α,β
-unsaturated esters", (Kwon Youn, Gyu Hwan Yon, Chwang Siek Pak, doi 10.1016/S0040-4039(00)84542-6)
There's an example in there with a p-methoxy cinnamate, so very promising.
It looks like a fairly straightforward methodology, so might give it a go in the near future.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
I have quantitatively reduced several methyl cinnamates with zinc dust and nickel chloride in methanol. The reaction is fast and workup is easy. The
products give negative tests with permangate and bromine reagents. There is at least one detailed paper on this procedure.
AvB
|
|
|
Mush
National Hazard
   
Posts: 632
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
Quality!
Some mp data
45 C
Elsinghorst, Paul W.; Gonzilez Tanarro, Camino M.; Gutschow, Michael
Joumal of Medicinal Chemistry, 2006, vol. 49, 25, p. 7540 - 7544
45-46 C
Hoshino et al.
Chemical and pharmaceutical bulletin, 1974, vol. 22, p. 1307,1311
boiling point
90 C 0.01 torr Semmelhack; Stauffer
Journal of Organic Chemistry, 1975, vol. 40, p. 3619
153-155C 1torr
Manske; Holmesf
joumal of the American Chemical Society, 1945, vol. 67, p. 95,97
Methyl-3.4.5-trimethoxycinnamat, CuH/2-Butanol reduction
Semmelhack; Stauffer
Journal of Organic Chemistry, 1975, vol. 40, p. 3619
Reductions with copper hydride. New preparative and mechanistic aspects
|
|
|
|