sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
Planned Polyethylene Glutamate Synthesis
A little background first: I've been wanting to make an anion-exchange membrane for a while now. I've been working on MysteriusBhoice's instructions
for a polyvinyl alcohol membrane crosslinked with glutamic acid, but I haven't finished it yet. Another idea I had is a polyester plastic,
polyethylene glutamate. It would basically be the same structure as polyethylene terephthalate, but using glutamic acid instead of terephthalic acid.
Glutamic acid has an amino group, which means that the polymer could act as an ionomer when treated with an acid, such as hydrochloric acid, or as a
permanent ionomer if I quaternized the amino group. There are a few (possible) advantages to this polymer. It would be a polyester, and polyesters
usually have low melting points, so it might be possible to melt or shape this polymer. It also shouldn't be water absorbent, so it wouldn't change
volume when wet, and it might be easier to prepare than crosslinked polyvinyl alcohol. I'll probably only have time to carry out this experiment in
about a week and a half, so I'm just looking for any preliminary suggestions at the moment.
Materials:
Monosodium Glutamate (MSG)
Citric Acid
Dilute Sulfuric Acid
Ethylene Glycol
Heat source
Distillation Apparatus
Procedure:
Dissolve the MSG in water, then add the appropriate amount of citric acid. This should precipitate glutamic acid. Wash the glutamic acid to
remove excess citric acid or sodium citrate.
Dissolve the glutamic acid in the appropriate amount of dilute sulfuric acid. This should ensure that both carboxylic groups and the amino group
are protonated, and leave the glutamic acid as the hydrosulfate salt.
Mix this solution with a slight excess of ethylene glycol.
Put this solution in the distillation apparatus, and heat it to a temperature moderately above the boiling point of water. Maintain this
temperature until the solution thickens.
Once the solution has thickened, I should be able to pour it into a shallow pan and let the plastic solidify from the solution. I'm the least
sure about this step, because I don't know in what phase the plastic will be, or how the ethylene glycol solution will affect it.
Separate the polymer (process to be determined when I see the final solution).
I'll definitely try this on a test tube scale before trying the full version, just to see how the polymer forms. Does anyone see any problems that
I've overlooked?
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Is citric acidic enough to protonate glutamic acid?
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
Apparently so, since MysteriusBhoice had good success using it. It seems that a relatively weak acid has to be used, because stronger acids create glutamic acid salts, which are more
soluble than glutamic acid.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Good success? He doesn't give a yield. When you titrate to the pI (isoelectic point) of glutamic acid with a strong acid you are good. Or just add an
equimolar amount of acid. Do recrystallize the acid as it will be impure.
[Edited on 21-6-2023 by Tsjerk]
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
I've tried titrating MSG with hydrochloric acid, and I never got a precipitate, so I'm guessing that my pH control isn't fine enough. How would I
recrystallize the acid? It seems to have very low solubility in water.
|
|
|
unionised
International Hazard
    
Posts: 5104
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
How will you know if it has worked?
How will you distinguish your product from copolymers with polyglutamic acid?
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by sceptic  | | I've tried titrating MSG with hydrochloric acid, and I never got a precipitate, so I'm guessing that my pH control isn't fine enough. How would I
recrystallize the acid? It seems to have very low solubility in water. |
When you don't get a precipitate my first guess is you used too much water. A concentrated MSG solution gives a very thick precipitation.
Recrystallization is done with hot water, with higher temperatures solubility goes up significantly.
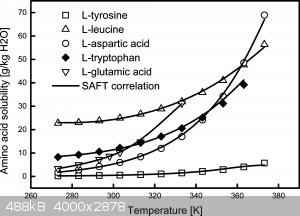
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
I hadn't thought about the formation of polyglutamic acid. To keep it from forming peptide bonds, I could quaternize the amino group on the glutamic
acid before step 3.
|
|
|
Kloberth
Harmless

Posts: 29
Registered: 2-6-2023
Member Is Offline
|
|
How would you quaternize the amino group. (After a quick gpt explanation I am now wondering how this could be done) I would find this project really
interesting and if the peptide formation can be prevented I would be keen to try this!
|
|
|
Texium
Administrator
       
Posts: 4516
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Making peptide bonds isn’t as easy as it looks. I doubt that it would be a significant issue under these conditions.
|
|
|
sceptic
Harmless

Posts: 49
Registered: 7-6-2022
Location: Southern Africa
Member Is Offline
|
|
I'll try acidifying a more concentrated solution of MSG. Thank you for the solubility data!
For quaternization, I'll probably try using diethyl sulfate or ethyl iodide. I haven't given that step much thought so far, but those seem like the
easiest alkylating agents to synthesise. Methyl iodide or dimethyl sulfate seem to be better documented, but I only have a little methanol.
|
|
|
KoiosPhoebus
Hazard to Self
 
Posts: 51
Registered: 23-1-2023
Member Is Offline
|
|
Quote: Originally posted by sceptic  |
Materials:
Monosodium Glutamate (MSG)
Citric Acid
Dilute Sulfuric Acid
Ethylene Glycol
Heat source
Distillation Apparatus
|
One comment I would add to this - most commercial MSG is a mix of 98%-99% monosodium glutamate, and 1%-2% disodium inosinate/disodium guanylate to
enhance the flavouring properties of the glutamate. Inosinic acid and guanosine monophosphate are more soluble than glutamic acid but I'm not sure how
strongly that holds at low pH - so just be aware that you might have some ribonucleotides in your product.
Quote: Originally posted by sceptic  | | Apparently so, since MysteriusBhoice had good success using it. It seems that a relatively weak acid has to be used, because stronger acids create glutamic acid salts, which are more
soluble than glutamic acid. |
That's not really true. They only create glutamic acid salts if you use an excess
of the acid, for example:
Na(Glutamate) + 2 HCl -> NaCl + GlutamateH2Cl
If you use a molar equivalent or slightly less than a molar equivalent of a strong acid then you're fine.
Quote: Originally posted by sceptic  | | I've tried titrating MSG with hydrochloric acid, and I never got a precipitate, so I'm guessing that my pH control isn't fine enough. How would I
recrystallize the acid? It seems to have very low solubility in water. |
The same thing initially happened to me. If you've added about 1 mole of HCl for
every mole of glutamate in solution, stirring will cause the formation of a white glutamic acid (and possibly ribonucleotide) precipitate which can
then be filtered off. The precipitate can then be washed with water to remove any sodium chloride.
|
|
|