| Pages:
1
2 |
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Cocrystalization
Cocrystalization is a very interesting phenomenon and i have seen not much about this subject on this forum.
From Wikipedia
Two explosives HMX and CL-20 cocrystallized in a ratio 1:2 to form a hybrid explosive. This explosive had the same low sensitivity of HMX and nearly
the same explosive power of CL-20. Physically mixing explosives creates a mixture that has the same sensitivity as the most sensitive component, which
cocrystallisation overcomes
The intermolecular interactions and resulting crystal structures can generate physical and chemical properties that differ from the properties of the
individual components. Such properties include melting point, solubility, chemical stability, and mechanical properties. Some cocrystals have been
observed to exist as polymorphs, which may display different physical properties depending on the form of the crystal.
For example, a mixture of hexamine diperchlorate and hexamine dinitrate at 1:1 will cocrystalize forming a new salt with different properties from the
original salts. I believe hexamine mononitrate monoperchlorate is created.
The same can be done with cations for example sodium potassium tartate. This is called double salt.
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Done with PETN and ETN it is very interesting I am told, no run aways like PETN and less sensitive then both if I remember correctly from another
member
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
Also, it's oxygen balanced with a VoD of around 8500m/s. The P/ETN mixture is probably one of the most powerful explosives easily attainable by the
amateur experimenter.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 386
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
I have browsed papers on cocrystallization but never looked into them. What is the technique? Recrystallizing with moles of each? If that's the case
are ternary crystallizations possible?
In searching im seeing a lot of ultrasonic solvent evaporation techniqes, it claims cocrystallization aside from previous mentionings in thread also
causes homogenous crystals largely free of defect for increased handling. Is ultra sonic necessary? What about just dissolving solvent with stirring
or using spraying of solvent on cold water surface or something similar?.
[Edited on 7-7-2023 by Hey Buddy]
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
It seems there is a larger proportion of CL-20 to HMX is the usual ratio to create the cocrystal structure.
Here is an excerpt from a 2022 paper that tested acetone, ethanol and ethyl acetate as solvents.
"The preparation of CL-20/HMX cocrystal was carried out at room temperature. First of all, 12 mL solvent was added into 1.1 g ε-CL-20 and 0.37 g
β-HMX with a molar ratio of 2:1 in glass vails. Then, cocrystals were prepared by evaporation of solvents at natural rate."
Solvent choice makes significant differences in the final crystalline product structure and properties and that paper found ethyl acetate to give the
best results.
Here is the link for it:
https://pubs.rsc.org/en/content/articlehtml/2022/ra/d2ra0373...
There are a few other papers out there, some using single solvents and some using dual solvent methods.
I have also read of other cocrystals of CL-20 with TNT and FOX-7. CL-20 has quite a remarkable ability to create these cocrystals and decent VOD to
go with it, it's a shame the cost to manufacture it is so high.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Cocrystalization seems very interesting, it may be also possible to create new solid oxidisers as rocket fuels. For example a cocrystalized AN/AP or
even AN/ Ammonium Chlorate. I do believe a cocrystalized salt of AN/AC can be made to be used as an oxidizers in rocket propellants. You may believe
that AC is too dangerous but the cocrystalized salt has complitely different characteristics from the original salt. For example 80/20 cocrystalized
AN/AC maybe superior to both since it may be more easily combustible be phase stabilized and be less hydroscopic (if it would be hydroscopic at all)
It will most likely outperform AP in ISP.
Even AN/AP cocrystalized would be better from AP since it will be cheaply more environmentally friendly (less chlorine gas) with better ISP since
Nitrogen is lighter than chlorine.
Also for energetics, the list is limitless
Guanidine/urea/biuret/diaminourea/ethylene diamine/piperazine/hexamine nitrate/chlorate/perchlorate/pursulphate/periodate/picrate/trinitrobenzoate and
the list goes on
Another interesting energetic would be cocrystalized Nitroguanidine with ETN
Below is an example of cocrystalized TNT with CL20
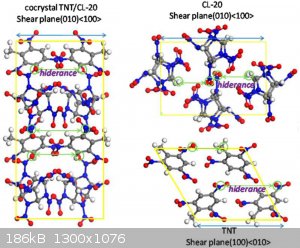
[Edited on 7-7-2023 by underground]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1339
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
It is also possible to melt ETN with nitrocellulose. After cooling, a solid, not brittle mass is formed. With a higher melting point. About 20% NC
increases the melting point to 90 Celsius. It has been tried. But I don't know the exact values and ratios and properties. The addition of camphor
comes into consideration. This could create ETN - celluloid.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
All nitro compounts, when its OB is relatively good, have a good performane close to 8k vod. The point is to find the easiest nitro compount to synth
and alter its properties with cocrystalization. To my mind the easiest nitrocompount are nitrourea, nitroguanidine. Maybe some other easier to synth
nitrate salts like biuret dinitrate and diaminourea dinitrate do the job. NU and NQ have good vod but have their drawbacks. The point is to remove
those drawbacks with cocrystalization in order to increase stability and apply castability. The point is cocrystalization increase stability while NU
NQ are quite insensitive already. So to my mind come cocrystalization with some sensitive nitro compounts like manitol hexanitrate and nitroclycerine.
Maybe even a cocrystalization with some primaries like NHN to decrease sensitivity. For example a cocrystalized energetic consisting mostly of NU/NG
with nitroglycerine/MHN/NHN to decrease sensitivity and melting point may be a good secondary. Note that some ions can cocrystallize together in any
ratio.
[Edited on 7-7-2023 by underground]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 386
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by underground  | All nitro compounts, when its OB is relatively good, have a good performane close to 8k vod. The point is to find the easiest nitro compount to synth
and alter its properties with cocrystalization. To my mind the easiest nitrocompount are nitrourea, nitroguanidine. Maybe some other easier to synth
nitrate salts like biuret dinitrate and diaminourea dinitrate do the job. NU and NQ have good vod but have their drawbacks. The point is to remove
those drawbacks with cocrystalization in order to increase stability and apply castability. The point is cocrystalization increase stability while NU
NQ are quite insensitive already. So to my mind come cocrystalization with some sensitive nitro compounts like manitol hexanitrate and nitroclycerine.
Maybe even a cocrystalization with some primaries like NHN to decrease sensitivity. For example a cocrystalized energetic consisting mostly of NU/NG
with nitroglycerine/MHN/NHN to decrease sensitivity and melting point may be a good secondary. Note that some ions can cocrystallize together in any
ratio.
[Edited on 7-7-2023 by underground] |
IMO NQ via GuNO3/H2SO4 is the simplest, mist time efficient per yield and resource efficiency of all nitros. I haven't found anything easier. Maybe
some of the stuff that requires no acid is easier NAP, TACP, TACN etc. But for high velocity nitro compounds NQ can be produced more efficiently and
quickly than most. I am always attracted to any useful advancement involving nitroguanidine for this reason alone.
|
|
|
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
I believe cocrystalization of NQ with a sensitive primary to requce sensitivity of NQ could end up in a very usefull secondary. A 3rd nitrocompound
can be added to that mixture to lower the melting point like TNT or ETN
I dunno if cocrystalized liquids with solids like EGDN and PETN after cocrystalization the evaporation of the liquids will stop. In theory they will
stop since the liquid EGDN crystalls are "trapped" into PETN crystal structure. With this way the pelting point of the cocrystalized would be reduced
drastically.
Cocrystalized NQ/Nitroclycerine may be very interesting.
Or NQ/ETN - NQ/MHN
[Edited on 7-7-2023 by underground]
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
"I dunno if cocrystalized liquids with solids like EGDN and PETN after cocrystalization the evaporation of the liquids will stop. In theory they will
stop since the liquid EGDN crystalls are "trapped" into PETN crystal structure. With this way the pelting point of the cocrystalized would be reduced
drastically.
Cocrystalized NQ/Nitroclycerine may be very interesting."
That is certainly an interesting concept if the two will behave together nicely but I think trying to obtain a cocrystal from a volatile liquid
explosive and a solid would be near impossible.
I would be led to believe that it would just form a mass of solid explosive that was "wet" with NG even after solvent evaporation. I
think that if there was any degree of co-bonding achieved there would be some weeping off the EGDN or NG out of the crystal's structure over time but
this would depend on the ratio and the quality of the bond achieved with the solid explosive crystal.
Or you could have the evaporation problem you stated and the liquid component leaves the crystal lattice over a period of time.
Another issue could be stress on the nitroglycerin during handling due to it being "trapped" in a hard crystalline mass. We know soaking NG into an
absorbent material reduces sensitivity but we have no idea of the properties when it is in a crystal lattice.
It would have great properties density and brisance-wise if it worked but I am quite sure you would just end up with a explosive oil coated energetic
powder.
NQ/MHN or NQ/ETN could be good though.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 386
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Out of what's been mentioned, ETN PETN and NQ, ANQ samples on hand if there's something worth testing... I plan to be in lab tonight testing nitrates
on proline and isoleucine...
|
|
|
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Hey Buddy  | | Out of what's been mentioned, ETN PETN and NQ, ANQ samples on hand if there's something worth testing... I plan to be in lab tonight testing nitrates
on proline and isoleucine... |
At first you could try to melt a 1/1 mixture of NQ/ETN to see if NQ get dissolved in ETN and at what temperature. You can then test its sensitivity
and if it can be detonated. If everything goes well we could then increase the NQ ratio to the point where still it could be detonated.
Another way of cocrystaloization is to dissolve both into a solvent and then evaporate the solvent. It has to be done in a way where when crystals
start to form, both crystals have to ppt out together.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
I tried an oxygen balanced melt of ETN/NQ 6/1 and the NQ does not dissolve in the ETN. I tested some that I made by melting them together, then
stirring it while it cooled and grinding it up. It has a higher VoD than pure ETN. The problem with combining them is ETN is soluble in alcohol and
insoluble in water, and NQ is the opposite.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Sir_Gawain  | | I tried an oxygen balanced melt of ETN/NQ 6/1 and the NQ does not dissolve in the ETN. I tested some that I made by melting them together, then
stirring it while it cooled and grinding it up. It has a higher VoD than pure ETN. The problem with combining them is ETN is soluble in alcohol and
insoluble in water, and NQ is the opposite. |
Those are some very good news. It was always a PITA to DDT NQ and it may be the 1st time someone actually DDT NQ at high density. With
cocrystalization NQ has its place. Cocrystalization can happen if both of them dissolved each other without any solvent. Have you figured out how much
NQ you can dissolve in melted ETN? Mixed solvents can also be made to dissolve both of them like acetone and water or find another solvent that can
dissolve both (maybe nitromethane)
The point is to find a sensitive explosive that NQ can be dissolved in it in large quantities. The goal is to use NQ as the main ingredient for a
cocrystalized secondary. NQ can be made easily with everyday chemicals (urea and ammonium nitrate) and it has a high VoD
Het Buddy found out that AspNO3 can dissolve a really big quantity of NQ. The main disadvantages of NQ is that it is a PITA to detonate and it is hard
to achieve high density. With cocrystalization high density can be achieved. The goal now is to find out a way to sentisize NQ
[Edited on 8-7-2023 by underground]
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Why not nitrate ETN with an excess of sulfuric acid and mix in an amount of guanidine nitrate. Meaning dump the ground ET and Guanidine nitrate mix
into the bath at the same time.
Wouldn’t that co crystallize?
My friend did the same for the ETN/PETN co crystal. Both were just dumped in a nitrating bath and a product that looked completely unlike PETN or etn
was produced in high yields.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
It might, but the product would have to be recrystallized and neither alcohol nor water would work. We need to find a solvent that can dissolve a
reasonable amount of both compounds.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
ETN/PETN can be dissolved in Acetone. Both are quite soluble in acetone
[Edited on 12-7-2023 by underground]
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
I meant for the NQ/ETN mixture.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
You can just melt each other without any solvent
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
I tried it, and NQ doesn't dissolve in molten ETN.
[Edited on 12-7-2023 by Sir_Gawain]
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
You can try mixed solvents too like water and acetone
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
I don't think it would work. NQ is pretty insoluble in acetone. A solution of NQ in hot water will precipitate upon addition to acetone.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
underground
National Hazard
   
Posts: 697
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
There are plenty of solvents out there. Use your imagination. Nithromethane possibly can dissolve both. Methanol ? Isopropyl? Mek? Toluene? Benzene?
|
|
|
dettoo456
Hazard to Others
  
Posts: 178
Registered: 12-9-2021
Member Is Offline
|
|
@underground NMP or another polar aprotic like DMF and DMSO might work. NQ has been verified to be soluble in NMP and DMSO and if the polarity trend
follows, then ETN might be aswell
|
|
|
| Pages:
1
2 |