Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Preparation of cyanogen bromide and a polymethine dye
Pyridine reacts with cyanogen bromide under formation of an adduct followed by ring opening and formation of glutaconaldehyde, which is called the
Zincke-König-reaction.[1] It can also be archieved by 2,4-dinitrochlorobenzene and pyridine,[2] from pyridinium-1-sulfonate and
NaOH,[3] or from pyridine and thionyl chloride via N-(4-pyridyl)pyridinium chloride.[4,5]
I was made aware of this dye by mgritsch from Illumina-Chemie.
Cyanogen bromide is made in situ from thiocyanate and bromine.
It should be stated very clearly that BrCN is very toxic and quite volatile. All experiments were
performed in a fume hood which was tested prior to the experiment with bromoacetone, boiling ammonia and bromine (no smell or irritation could be
noticed). I was working with a lab partner for this and had a strong NaOH-solution and bleach on hand. Cyanogen bromide at least has good warning
properties, being extremely irritating prior to lethal concentrations. Due to the volatility of BrCN, the flask was kept stoppered as much as
possible, and gloves were worn. No irritation was felt during the reaction. But do not attempt this if you don't have the safety precautions in place
and don't know what you are doing.
All reagents were weighed out before starting the reaction.
Sodium thiocyanate
A NaSCN solution was prepared by boiling 2.5 g of NH4SCN, 1.31 g of NaOH and 20 g of water until no more ammonia could be detected using
wet pH-strips. Water was added for a total volume of about 20 mL. While the formation of BrCN also works with ammonium thiocyanate, on neutralizing
the solution it would create cyanamide via BrCN + 2 NH3 → NH2CN + NH4Br. If NaSCN or KSCN are available, they would
be preferred.
Cyanogen bromide[8]
NaSCN + 4 Br2 + 4 H2O → BrCN + NaHSO4 + 7 HBr
In a 500 mL flat bottomed flask were put 15 g of NaBr and dissolved using 100 mL of water. The bromide serves to increase the water solubility of
bromine and to reduce its vapor pressure. A stir bar was added and the solution cooled in an ice bath (the following reactions are
exothermic). To this flask were added 15.5 g of bromine (the bromine was previously distilled, dried over sulfuric acid and stored in
the freezer) and the mixture stirred until everything dissolved.
Then the thiocyanate solution was added dropwise to the bromine. It is very important that the thiocyanate is added to the bromine and not
vice versa! Bromine needs to remain in excess for the reaction. The thiocyanate is dropped/titrated in slowly with stirring just until the
solution turns colorless - an excess was prepared beforehand, so not all solution is used. (Theoretical required amount of thiocyanate: 24.2 mmol,
1.966 g NaSCN or 1.846 g NH4SCN)
At this point the flask contains an acidic aqueous solution of cyanogen bromide. While it can be extracted with organic solvents, this seemed too
dangerous for me.
Pyridine ring opening
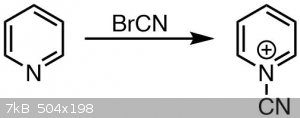
Whether the glutaconaldehyde derivative, that is the ring opened pyridine, is formed here already I am not sure of. Given the yellow color I am
led to believe it is. If so, the byproduct is cyanamide and produced in this step.
Then, one drop of a 1% solution of phenolphthalein in isopropanol was added. Then a previously prepared solution of 7.8 g (theoretically required:
7.760 g) of NaOH in 20 mL of water is slowly dropped in until the phenolphthalein just turns pink (should happen around pH 8.2). The goal is to
neutralize all the formed acid while not making the solution too basic, as base destroys BrCN.
Then, 3 g of pyridine are added with a pipette. The solution instantly turns a dark yellow, then orange, then red. In a previous experiment I was made
aware of the volatility of BrCN in a dramatic way by the fact that the pyridine I held in a beaker next to the mouth of the just unstoppered flask
turned yellow - it reacted with BrCN vapor. After all the pyridine was added, the solution was stoppered and stirred for 10 minutes, the ice bath was
removed and the solution stirred for a further 10 minutes. It has attained a more red-brown color.
Dye formation
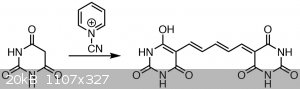
The byproduct is cyanamide. Alternatively written with the glutaconaldehyde. The dye is possibly in the form of the sodium salt.
Then, a solution of 3 g of barbituric acid in 35 mL of water was heated to about 60-65 °C until it dissolved - this solution is practically
saturated. It is added in one go to the flask, which is then restoppered and stirred for 45 mins at room temperature. Initially no reaction is
visible, but the solution slowly turns black. After having stirred for 45 minutes, it is filtered through a filter paper. The solution contains traces
of cyanide (presumably as HCN due to the pH) and potentially some unreacted BrCN, so care must be taken. The majority should be in the form of
cyanamide and its degradation products however. The precipitate is washed several times with water. It is quite insoluble, despite the washing water
being quite strongly colored. The filter paper turns a lovely purple color.
The precipitate is air dried in the fume hood. The filtrate is basified with strong NaOH and the pH is confirmed using test strips, and left to stand
stoppered for a day in the fume hood. Only a very weak blue reaction is obtained by acidifying a sample of the solution again and adding Fe(II) and
Fe(III) salts (test for cyanide by formation of prussian blue). The solution is therefore carefully rinsed down the drain with a large volume of water
and more water is used to flush it down the pipes.
The yield of the dye is 1.437 g (~17 %). It is a black powder and looks like charcoal.
When a tiny amount is dissolved in some water, which it does only reluctantly, a red-purple solution is obtained. It is purple when backlit, but shows
red fluorescence under white (LED) light. It does not show fluorescence under 365 nm UV.
The compound is interesting because it acts as a sensitive test for magnesium ions.[5] In basic solution a blue precipitate occurs and the
fluorescence disappears if magnesium is present. (I seem to have forgotten to take a picture of it.)
I am not completely sure where most of the loss of yield occured. The initial reaction to BrCN is quantitative, but maybe there were ammonia traces
left that formed cyanamide, maybe the reaction with the pyridine wasn't complete, maybe some hydrolysis occured when neutralizing the reaction mixture
or the final reaction to the dye doesn't work that well under these conditions. Supposedly, cyanogen chloride is even better (about 30x when used for
the photometric determination[6]) for the formation of the dye than cyanogen bromide is, so some yield loss could also have occurred there.
(I also came across a variation that uses thiocyanate, chloramine-T and an iron(III)-salt as catalyst for the formation of ClCN
afterwards.[7]) I am also not completely sure if the pH of the solution at the end is optimal for the precipitation of the dye.
But it should also be pointed out this is quite a complex reaction sequence with many things happening in one pot.
In a previous attempt the reaction mixture was supposed to be neutralized with sodium acetate. However, not enough was added, so the solution was
still very acidic. The pyridine attained the same color, but the dye formation did not work, and only a beige, off-white product could be recovered.
Presumably it was just barbituric acid, perhaps bromobarbituric acid.
While the dye might not have given a good yield, the direct preparation of BrCN from thiocyanate is apparently quantitative, and is something I
haven't seen mentioned here.
Literature:
[1] - E. Asmus, HF. Kurandt, Einige analytische Anwendungen der Zincke-Königschen Reaktion. Forschungsberichte des Wirtschafts- und
Verkehrsministeriums Nordrhein-Westfalen, VS Verlag für Sozialwissenschaften, Wiesbaden 1958, 495, 6. https://doi.org/10.1007/978-3-663-04788-9_1
[2] - P. Baumgarten, Ber. dtsch. Chem. Ges. A/B 1924, 57, 1622-1627. https://doi.org/10.1002/cber.19240570876
[3] - J. Becher, Org. Synth. 1979, 59, 79, https://doi.org/10.15227/orgsyn.059.0079, http://orgsyn.org/demo.aspx?prep=CV6P0640
[4] - E. Koenigs, H. Greiner, Ber. dtsch. Chem. Ges. A/B 1931, 64, 1049-1056. https://doi.org/10.1002/cber.19310640518
[5] - V. Anger, Mikrochim Acta 1961, 49, 512–515. https://doi.org/10.1007/BF01217485
[6] - E. Asmus, H. Garschagen, Z. Anal. Chem. 1953, 138, 404–414. https://doi.org/10.1007/BF00461092
[7] - E. Asmus, H. Garschagen, Z. Anal. Chem. 1953, 138, 414–422. https://doi.org/10.1007/BF00461093
[8] - W. König, J. Prakt. Chem. 1911, 84, 558-560. https://doi.org/10.1002/prac.19110840139
Pictures:
Some of the images are from that previous attempt, which is why the ice bath is missing in the first images. It was added in the second run because
the solution got quite warm.
 
Titrating in the thiocyanate. Left: Bromine. Right: Complete addition.

The color of the reaction mixture when all pyridine is added. Initially it is very yellow.

After addition of barbituric acid and some time stirring, the solution contains the dye in small particles.

Filtration of the dye.

The interesting color of a very dilute aqueous solution of the dye. Left: Backlight. Right: Direct light.
we apologize for the inconvenience
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 163
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
Excellent work and write-up - great pictures too!
You're a lot braver than I am, I'd be loath to work with cyanogen anything.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Very nice rite-up Diachrynic. You beat me to it! I have been looking into this reagent for magnesium too but I was planning on using the
2,4-dinitrochlorobenzene route to the pyridine ring opening as I have lots of this compound and it is much easier to handle than cyanogen bromide
although the procedure sounds a bit messy (I translated Zinke's original work on the reaction of 2,4-dinitochlorobenzene with pyridine, very
interesting). I could use the thionyl chloride route. I am certainly tempted to try this preparation though perhaps via a different glutaconaldehyde
precursor.
In the end I plumbed for a different reagent for Mg, N,N-Bis(salicylidene)-2,3-diaminobenzofuran which is prepared by a slightly less onerous
preparation from salicylaldehyde, ammonia and an alkali cyanide. It gives an intensely orange fluorescent complex with Mg2+.
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Diachrynic great !!!
Do you think the reaction could be performed also with thiobarbituric acid? I have both barbituric as well thiobarbituric acid. Thio could have
different color (e.g. indigo is blue and thioindigo red)
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Thanks Lionel, Boffis and Fery!
Boffis: The thionyl chloride route does work, it's what my friend used, but I don't think he published a write-up anywhere yet... Also wow, that other
compound looks very interesting, thanks for sharing!
Yes, pretty sure. Paper [5] (V. Anger, Mikrochim Acta 1961, 49, 512–515. https://doi.org/10.1007/BF01217485) states that the condensation product of thiobarbituric acid and glutaconaldehyde is "in aqeuous acetic acid
solution blue, in alkaline solution gree with green fluorescence and in mineral acid red, forms no magnesium salt but in weak nitric acid solution
purple salts with silver, mercury(I) and copper(I)". It could be more soluble than the one with regular barbituric acid though, I haven't looked
further yet.
we apologize for the inconvenience
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Diachrynic thanks for useful link!
Concerning thionyl chloride + pyridine I found only this, in one page with the cyanogen bromide:
https://books.google.cz/books?id=RabhBwAAQBAJ&pg=PA321&a...
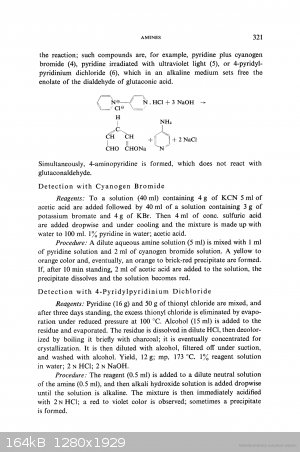
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
I think it might be possible to circumvent the cyanogen bromide by treating the pyridine in acetonitrile with a little water added with NBS, what will
brominate the nitrogen and then adding ZnCN (NaCN and KCN will work but with lower yields). Reaction with ZnCN is heterogeneous but this does not
hurt.
It is described for different substrates in the attached article, courtesy MrBovinejony
Attachment: Synthesis of Cyanamides NBX + MCN.pdf (871kB)
This file has been downloaded 128 times
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Has anyone ever tried the pyridyl-pyridinium salt as a source of glutaconaldehyde? How would you remove the 4-aminopyridine by-product? Can you just
filter it off as its sparingly soluble in alkaline solutions as you can with the 2,4-dinitrochlorobenzene route of Baumgarten (2,4-dinitroaniline in
this case). Since I have lots of dinitrochlorobenzene and a small amount of 4-pyridylpyridinium dichloride I am planning on trying one of these
routes.
By the way has anyone read reference 4 above by Koenigs and Greiner? When I made my pyridyl-pyridinium dichloride and mixed pyridine with thionyl
chloride I was expecting copious emissions of sulphur dioxide but I got virtually no gas released at all and the reaction is only slightly exothermic
so I read Koenigs' paper and the mechanism of the reaction is clearly very obscure, they offer no real proposal for the mechanism of formation of the
pyridyl-pyridinium unit.
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
I randomly stumbled across A. Ledwith, P. J. Russell, J. Chem. Soc., Perkin Trans. 2, 1974, 582-591, https://doi.org/10.1039/P29740000582. In this paper, they produce the pyridylpyridinium salts using pyridine and persulfate. This is much more
accessible than the thionyl chloride route. The conditions seem quite easy. Perhaps this could be used as a one-pot reaction to form the polymethine
dye too.
[Edited on 29-11-2023 by Diachrynic]
we apologize for the inconvenience
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Good finding Diachrynic !!! More available route! Interesting that the Na2S2O8 gives that dimer by a radical mechanism while H2O2 reacts with pyridine
into pyridine-N- oxide.
|
|
|
|