DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Nitration of Tetramethylene dinitroethane tetranitrate
There is a very potent nitroether(Tetramethylene dinitroethane tetranitrate, (O2NOCH2)2C(NO2)C(NO2)(CH2ONO2)2), which can also be processed by
casting. It is a yellowish crystalline substance, insoluble in water, well soluble in alcohol.
The density is 1.917 g/cm. Melts at 85-85°C, decomposition begins at 141°C. Power at the level of HMX (Detonation velocity 9100 m/s, pressure 400
Kbar).
There is a patent for its synthesis on the net. And I have raised questions about the third stage of synthesis. I'll give it here:
«The preparation of compound 1 was as follows: Acetic acid (50 mL) and acetic anhydride (50 mL) were added to a200 mL jacketed flask. The
solution was then cooled to 0° C. and nitric acid or HNO3 (34 g, 98%) was added dropwise while maintaining the reaction temperature below 5° C. The
reaction was allowed to stir for 20 minutes and 4 (12 g, 0.05 mol) was added portionwise. After stirring the mixture for 2 hours at 0° C., the
temperature was raised to 208° C. over one hour and then stirred at 208° C. for an additional hour. The reaction mixture was then poured into 200 mL
of ice-water and stirred. The white solid was filtered, washed with water, and air dried to give of crude 1 (20 g). This material was then
recrystallized from isopropanol to give 18 g of 1 (85%). M.p. 85-86° C»
What does the phrase "nitric acid or HNO3" mean? )) Okay, that could be a tautology. But here's "After stirring the mixture for 2 hours at 0° C., the
temperature was raised to 208° C. over one hour and then stirred at 208° C. for an additional hour." How's that? Because the compound already
decomposes at 141° C. And how will the nitro mixture behave at such a high temperature? I know for sure that trinitrobenzene can be obtained from
dinitro by nitration with 100% nitric acid and 160% sulfuric acid (oleum). It is nitrated at 120° C for 6 hours, and the yield is 65-70%. But 120° C
is not 208° C. And aromatic nitro compounds in general are much more heat resistant than nitroesters.
What do you people think, could this be a typo in the patent (accidental or intentional) and actually meant 20.8°C? But in that case it is strange to
specify the decimal part, there is almost no difference.
Just in case, I will give the formula of the compound and the patent.
Here on the forum I have not found information on this compound. But by the way, maybe I did not search well.... then I apologize in advance
P.S. By the way, it is not clear what density the substance has in cast form. And it is important)))
For example, picric acid has a density of 1.813, but cast only 1.61 (however, it is not critical for it). The same ETN on the contrary is very good in
the cast form, with a density of 1.72 has a cast melt of 1.7. But such a substance as TNAZ (trinitroazetidine) has a density of 1.84, but in the cast
form of 1.55-1.59. It will be very sad if this nitroether in the cast form does not give at least 1.8. Then it is easier to make ETN.
Attachment: US8324421.pdf (353kB)
This file has been downloaded 111 times
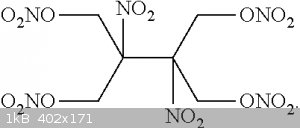
[Edited on 7-9-2023 by DennyDevHE77]
|
|
|
PLSHY
Hazard to Self
 
Posts: 88
Registered: 30-7-2023
Member Is Offline
|
|
This has great potential! If the casting density can reach 1.9, then this will be the strongest explosive for amateurs. Has anyone tested the
density of inositol nitrate? Because myo-inositol already has a density as high as 1.75, the density of myo-inositol nitrate is likely to reach above
1.9.
|
|
|
Etanol
Hazard to Others
  
Posts: 138
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DennyDevHE77  |
After stirring the mixture for 2 hours at 0° C., the temperature was raised to 208° C. over one hour and then stirred at 208° C. for an additional
hour.
|
It's nitroester. Read as 20,8° or 20°C.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Thank you. I'm glad I wasn't alone in my suspicions that there was a trivial mistake, a comma missing.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PLSHY  | | This has great potential! If the casting density can reach 1.9, then this will be the strongest explosive for amateurs. Has anyone tested the
density of inositol nitrate? Because myo-inositol already has a density as high as 1.75, the density of myo-inositol nitrate is likely to reach above
1.9. |
Some here on the forum have nitrated him before. But they write that it is comparable in sensitivity to HMTD and does not keep for a long time. At
Microtek it decomposed after several months. This is not surprising, the same mannitol hexanitrate lives about half a year if you do not use
stabilizers like centralites.
http://www.sciencemadness.org/talk/viewthread.php?tid=9201
|
|
|
Microtek
National Hazard
   
Posts: 828
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Sensitivity of NEST-1 (the name the authors have given it) should be on par with PETN, not HMTD.
When I made it, I did not investigate possible ways to stabilize the compound, so it is possible that something could be done to increase shelf life.
I found that nitration of the parent molecule could be accomplished equally well using H2SO4/HNO3 or HNO3/P2O5. I confirmed the identity of the
product by spectroscopy in all cases (sadly, I don't have access to any spectroscopic equipment any longer).
I'm attaching the paper that the patent is based on.
Attachment: Synthesis of an Energetic Nitrate Ester.pdf (376kB)
This file has been downloaded 116 times
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Wait, are we talking about innositol hexanitrate or about this NEST-1 about which I made a topic? In the previous message I meant innositol
hexanitrate, and there I also referred to your comment that it decomposed in you within several months.
|
|
|
Microtek
National Hazard
   
Posts: 828
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Well, I'm talking about NEST-1. It's true that I made inositol hexanitrate many years ago, but I didn't experiment with it very much. I think I
aquired pentaerythritol about that time and simply transitioned away from MHN and IHN before doing much.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Microtek, I once wondered why in the scientific literature most nitroesters are obtained using sulfur-nitrogen nitro mixtures. Why doesn't anyone use
phosphoric anhydride? I thought just because of the price, but I have encountered opinions that nitro mixtures based on nitric acid + phosphoric
anhydride are too active for esters. The only thing I found is that starch and cellulose give the highest nitrogen content when nitrated with P2O5 or
phosphoric acids.
But you have been quite successful using P2O5 to synthesize NEST-1. Can nitroglycerin, ETN, PETN, EGDN be obtained in this way? There is nothing
described in the literature.
It just looks very attractive. All you have to do is buy P2O5 and 65% nitric acid. Mix them together in the cold and you're good to go. No long-term
distillation, no assembling and disassembling of the distillation apparatus, etc.
|
|
|
Microtek
National Hazard
   
Posts: 828
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I think the main reason that P2O5 is not used more than it is, is that it is difficult to work with. Even in labs with low relative humidity, the
powder will clump up and form sticky lumps during addition. In an industrial setting it would probably be even worse. It is usually possible to use a
mixture of P2O5 and ordinary phosphoric acid instead. This is a liquid, and thus more easily handled, but it doesn't scavenge water as well as
straight P2O5.
In order to convert all the water in 65% nitric acid to phosphoric acid you would need to add about an equal mass of P2O5. It would evolve enormous
amounts of heat, and the nitric would be fairly dilute (with phosphoric acid), which may or may not be a problem. I think P2O5 is better saved for
when you need N2O5 as the nitrating species, or when you have 95% nitric and want to remove the last of the water.
|
|
|