PLSHY
Hazard to Self
 
Posts: 88
Registered: 30-7-2023
Member Is Offline
|
|
"DINA", the nitration product of diethanolamine
I have heard of it a long time ago, but recently I came across it in a book. As an amateur energetic material, it has the following advantages.
1. It can be cast (melting point 50 degrees), this is the best advantage! Because I didn’t think of anything more suitable for shaped perforating
bombs than casting!
2. It is powerful, at least 150% of the power of TNP (lead mass test), with an explosion velocity of more than 7700 at a casting density of 1.67.
3. Lower cost, if you have fuming nitric acid, it can be manufactured at a lower cost than petn, and the purification process is easy.
4. The mechanical sensitivity is low. For the above three advantages, etn can do better than it. But this advantage separates it from etn. It has a
similar sensitivity to tetryl, which means it can be cast in a safer manner.
But similarly, he also has the following shortcomings.
1. The stability is poor. The stability test at 72 degrees Celsius lasts for more than 30 minutes, but the decomposition temperature is only 160
degrees, and the stability is much worse than tetryl.
2.It can only be made with fuming nitric acid. Due to the special nitramine structure, it can only be made with nitric acid with a mass fraction
greater than 95%.
3. There is less information. For energetic material enthusiasts, information on a substance is very important, but there is less information on dina.
Overall, this is a very nice substance. It is possible to replace etn as the first choice for amateur casting and casting explosives, greatly
improving safety. And due to its high energy, it can also be used as a component of high-energy explosives
[Edited on 2-11-2023 by PLSHY]
|
|
|
PLSHY
Hazard to Self
 
Posts: 88
Registered: 30-7-2023
Member Is Offline
|
|
What interests me the most is its simple production method. For enthusiasts, synthesizing some petn is always easier than synthesizing tnt. But the
cost of TNT in industry is lower than PETN. For dina, all you need is some diethanolamine, fuming nitric acid, sodium chloride and magnesium oxide.
The ratio is approximately 1:4.5:0.032:0.4. And all you have to do is mix them together! It’s almost as simple as petn’s nitrification. There is
no need for a lot of glass instruments and complex three-stage nitrification waste acid recovery!
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
As far as I know, acetic anhydride is needed for the production of DEAN, without it it turns out (O2NOCH2CH2)NH*HNO3. To separate water from amine,
acetic anhydride is needed, well, maybe phosphoric will do.
|
|
|
PLSHY
Hazard to Self
 
Posts: 88
Registered: 30-7-2023
Member Is Offline
|
|
Quote: Originally posted by DennyDevHE77  | | As far as I know, acetic anhydride is needed for the production of DEAN, without it it turns out (O2NOCH2CH2)NH*HNO3. To separate water from amine,
acetic anhydride is needed, well, maybe phosphoric will do. |
There is a method that does not require acetic
anhydride and is called the magnesium nitrate method.

|
|
|
EF2000
Hazard to Others
  
Posts: 109
Registered: 10-5-2023
Location: The Steppes, now trapped in the forest zone
Member Is Offline
Mood: wrooom
|
|
Then the main problem is DEA itself. It's widely used in things like cosmetics, but is it available OTC? I checked chemcraft, they have only
triethanolamine (TEA) and ethanolamine O-sulfate, though I haven't searched for other suppliers
Wroom wroom
|
|
|
PLSHY
Hazard to Self
 
Posts: 88
Registered: 30-7-2023
Member Is Offline
|
|
In China, you can purchase analytically pure reagents through 999 Chemical Network, which is equivalent to about US$5 for a 500ml bottle. I also know
that it is available on the Chinese chemical website "1688" at a very cheap price. As far as I know, diethanolamine is a bulk industrial product and
may be easier to find on chemical trading websites. It has many uses such as lubricant, coolant, corrosion inhibitor.
|
|
|
dettoo456
Hazard to Others
  
Posts: 178
Registered: 12-9-2021
Member Is Offline
|
|
https://doi.org/10.1021/acs.oprd.9b00300 -This paper outlines the Ac2O/HNO3 method but PLSHY is correct in that there are other methods to
produce it cheaply. The DEA is cheap too, but sourcing is a little more tricky than finding MEtOHA or TEtOHA. If making though, it is paramount to
nitrate all the way to the nitramine (not just the nitrate di-ester) because there are apparently much larger stability issues with the free amine.
Stability is an issue though; as cheap as the DINA may be, pentaerythritol is cheap too (around $1.5/lb) and PETN is far more stable, easy to
synthesize, easier to handle, and more powerful. If you want a cheap, stable liquid EM I’d just recommend a simple ammonium salt like ethyl- or
methylamminium nitrate or a cyclic nitrate ester like IHN. Now that sugar-free sweeteners are becoming more popular, the market is bigger than ever
for cheap polyols for nitration.
|
|
|
Microtek
National Hazard
   
Posts: 828
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I think the appeal is that DINA is melt castable unlike PETN and most of the nitrate esters. If you look at the NENO thread, the strategy there is to
convert the primary amine to a secondary one which is more easily nitrated without forming salts. NENO does require quite harsh conditions to nitrate
fully though.
|
|
|
EF2000
Hazard to Others
  
Posts: 109
Registered: 10-5-2023
Location: The Steppes, now trapped in the forest zone
Member Is Offline
Mood: wrooom
|
|
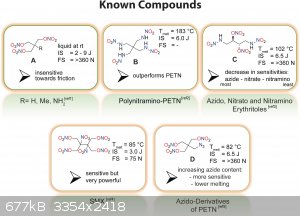
That's true, and DINA is interesting for sure, but there're melt castable derivatives of PETN: https://doi.org/10.1002/chem.202204013
Wroom wroom
|
|
|
Microtek
National Hazard
   
Posts: 828
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
But they are much more difficult to prepare than DINA (at least the ones in that paper)...
I did see references to pentaerythritol TRInitrate once. I forget the energetic properties, but I would guess in the vicinity of NG. I could imagine
that it would be quite difficult to only nitrate three of the hydroxy groups, though.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
I found a mention of trinitrate on one dead forum.
Quote from one of the participants:
"There is an interesting field for pyromaniac activity for those who like to get involved with pentaerythritol.
For those in the tank: Petrin is a viscous liquid with a solidification temperature of about 20°C and a density of 1.54 g/ml.
The ability to plasticize nitrocellulose is superior to nitroglycerin, while only slightly losing to it in power is much less sensitive (like tetryl)
and less poisonous, because it penetrates the skin worse and is less volatile. In the USA, at one time, all kinds of plastisols were made on it and
used in gunpowder instead of nitroglycerin...
In general, how it is done (recipe from JACS 1955): 480g of pentaerythritol for 10 minutes is added to 1980 g of 80% concentration of nitric acid
cooled to 0°C
(with the addition of 3 g of urine). During the addition and after, make sure that the temperature does not become higher than 5°C.
After 15 minutes of stirring, add 1980g of 80% sulfuric acid, cooled to -10°C. The mixture is continued to be stirred for 2 hours.
The mixture is poured into half a kilogram of ice and the sediment is filtered out. Wash it with water.
After that, the precipitate is dissolved in 2.5 liters of boiling acetone containing 75 g of ammonium carbonate.
The acetone solution is diluted with water and alcohol to a mixture containing 7 hours of acetone, 3 hours of water and 2 hours of ethanol.
After an hour of holding the mixture, the dropped PETN (!) is filtered out. Another 7L of water is added to the filtrate and left for 16 hours.
The water layer is decanted and the organic layer is filtered out. The precipitate is combined with the resulting PETN and used for its intended
purpose 
The filtrate is diluted with 1 liter of water and mixed well. The liquid organic layer is separated again.
Since vacuum distillation for household chemicals is often exotic, then in my opinion it is necessary to pour the mass into a saucepan, cover the pan
with gauze and take
it outside for a couple of days so that any volatile stuff evaporates, after which we dry it with anhydrous magnesium sulfate or calcium chloride.
The result is 450g of petrine, which has a 47% yield... Not counting the side-highlighted PETN" End quote.
There are other ways, for example, to obtain trinitrate through mono-acetate, but it is in turn prepared from tetraacetate, and here acetic anhydride
is already needed, and not every household chemist has it.
And someone even successfully obtained PETRIN by saponification of PETN with sodium hydroxide in alcohol.
By the way, penta monoacetate-trinitrate has a melting point of 87°C and probably also forms eutectic with PETN. Monoformate-trinitrate generally
melts at 60°C.
|
|
|
EF2000
Hazard to Others
  
Posts: 109
Registered: 10-5-2023
Location: The Steppes, now trapped in the forest zone
Member Is Offline
Mood: wrooom
|
|
Read as "3 g of urea".
And "JACS 1955" is that paper: A New Synthesis of Pentaerythritol Trinitrate (full text).
Cool method, gives you both desired liquid explosive and PETN as pleasant bonus. 404 g of PETN, actually, not much less than main product.
Was it on some Russian/Eastern European forum? "For those in the tank" idiom sounds very familiar.
Note: 80% acid concentration is very important:
| Quote: |
The use of 90% nitric acid gave almost quantitative yields of pentaerythritol tetranitrate while a slight reduction in concentration of nitric acid
gave negligible quantities of both pentaerythritol tetranitrate and trinitrate. |
[Edited on 8-11-2023 by EF2000]
Wroom wroom
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Yes, it's from the Russian forum "exploders.info". It's dead now, and is only available in the archive. And the specific article is here: http://web.archive.org/web/20160418231346/http://www.explode...
I'll note that it opens far not always and not immediately, apparently because of problems on the web archive itself. It is not commercial and lives
on donations.
I apologize for the urea, I translated it just like that, although it was mentioned as a metonymy.
|
|
|