Niklas
Harmless

Posts: 26
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Synthesis of 9,9‘-bianthracene from anthraquinone
Hey everyone, just created this account, and to start I wanted to share this small experiment I carried out quite a while ago (unfortunately I
didn’t take too many pictures tho, and the ones I did take honestly aren’t the best quality either..), which is the synthesis of
9,9‘-bianthracene, may be better know as 9,9‘-bianthryl to some of you, from anthraquinone, using tin granules and fuming hydrochloric acid in
glacial acetic acid as the solvent, going through a Clemmensen reduction, a Pinacol coupling and an E1-elimination all in one step.
The procedure itself actually is quite straightforward, and probably something most of you wouldn’t even have to get any new reagents / equipment
for, making it quite a neat compound to synthesize if you are looking for a somewhat exotic luminophor.
Required chemicals:
- 6 g of anthraquinone
- 24 g of tin granules
- 72 ml of 100% acetic acid
- 36 ml of 37% hydrochloric acid
- some 7% hydrochloric acid
- some toluene
Experimental procedure:
To a 250 ml three-neck RBF, equipped with a Dimroth condenser and a small addition funnel, all the anthraquinone and tin metal granules were added.
Next all the glacial acetic acid was poured in and the solid components were brought into suspension using a magnetic stirrer.
The condenser was flushed with cold water and heating was turned on to bring the mixture to a reflux. Meanwhile the fuming hydrochloric acid was
filled into the addition funnel, and as soon as things got to temperature it was added really slowly over the course of about one hour.
The reflux was continued for another four hours (a color change from yellow to olivegreen was observed within this time period), the apparatus was
disassembled, and, while still hot, the reaction mixture was decanted into a beaker, leaving behind and unreacted tin.
After cooling back down to room temperature the product suspension was filtered using vacuum filtration and the resulting slightly yellow powder was
washed with multiple small portions of 7% hydrochloric acid, so that residual any tin(II)-chloride, occurring as a major side product, can be removed.
After drying on the pump for a few minutes, a simple recrystallisation from hot toluene was performed, yielding some reasonably pure product that
exhibits a bright blue fluorescence when irradiated with UV.
Yield: 2,93 g; 57% of the theoretical
To enable a further functionalisation of the resulting hydrocarbon a bromination using NBS in carbon tet could be performed, yielding
9,9‘-bi-(10-bromoanthracene).
I hope this small post contained some interesting information for you, would be keen to see other ones try the synthesis out too, but for now, have a
good one ya‘ll!
  
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Niklas welcome here. Great experiment, beautiful fluorescence!!!
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Niklas, welcome to the madhouse. Nice preparation and write up for this interesting compound. I wonder if you could prepare this compound by an
Ullmann type reaction from 9-bromoanthracene. I ask as I have a lot of 9-bromoanthracene and no anthraquinone, I started a thread about how to utilise
this compound, I envisaged de-halogenation and then oxidation to anthraquinone. I am worried that if I simply try oxidation directly on the
bromo-compound I will generate brominated anthraquinones due to the liberated bromine.
On the other hand 9-bromoanthracene might just suffer the same fate as anthraquinone. Hmmm might just have to try this (thinking aloud).
|
|
|
Niklas
Harmless

Posts: 26
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Would probably be another possible path, yeah. Performing the reaction in cumene under addition of thiophene-2-carboxylic acid would likely give the
best results, since this way the temperature required for an Ullmann could be reached more easily.
Also thanks for the welcome!
|
|
|
Niklas
Harmless

Posts: 26
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Quote: Originally posted by Boffis  | | I ask as I have a lot of 9-bromoanthracene and no anthraquinone, I started a thread about how to utilise this compound, I envisaged de-halogenation
and then oxidation to anthraquinone. |
If you still wanted to turn on into another first tho, maybe for another project, then performing the dehalogenation using sodium in t-butanol [1] and
the oxidation using sodium / potassium chlorate [2] should work quite well I believe.
[1] https://patentimages.storage.googleapis.com/af/d5/67/c77d64d...
[2] https://www.chem-page.de/synthesen/anthrachinon.html?highlig...
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
The procedure is taken from "The Symmetrical Dianthryls. Part I" by Bell and Waring. It is attached - for the interested.
ps.
a patent (US20120065445A1) gives very similar procedure but with zinc instead of tin.
Attachment: JR9490000267.pdf (97kB)
This file has been downloaded 104 times
Слава Україні !
Героям слава !
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
That old JCS paper is an interesting find as it give several other routes including a Grignard reagent route but then states that the
9-bromoanthracene doesn't react with sodium even in boiling xylene! But I will still have a go at an Ullmann type reaction with copper powder and
de-bromination with t-butanol and Na. Does anyone think that lithium would give 9-lithium anthracene?
@Niklas, thanks for the link to the anthraquinone prep! I must admit that I would not have thought about a chlorate in this context as I would have
expected this to lead to some chloroanthraquinone side products.
|
|
|
Niklas
Harmless

Posts: 26
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Probably, since halogen-lithium exchanges should generally work quite well on aryl compounds like this one
|
|
|
Niklas
Harmless

Posts: 26
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Quote: Originally posted by Boffis  | | @Niklas, thanks for the link to the anthraquinone prep! I must admit that I would not have thought about a chlorate in this context as I would have
expected this to lead to some chloroanthraquinone side products. |
No problem! Always like to share this synthesis approach for anthraquinone from anthracene, since it‘s much safer and also more accessible for most
home chemists compared to the well known CrO3 route
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Yesterday I ran a "quick and dirty" experiment with 9-bromo-anthracene and copper bronze. I mixed 5.005g of bromo-anthracene with 3.001g of copper
powder (an excess x2.4 molar ratio), ground them together to get an intimate mixture. A small sub-sample, circa 1g, was separated and placed in a 12mm
ID pyrex test-tube and then heated in a bunsen flame until the mixture boiled freely. The molten mixture was maintained at this temperature for about
10 minutes, the mixture became dark brown and a little yellow sublimate formed higher up the tube that had to be melted down occasionally. The
test-tube was cooled and then toluene was added, 1ml at a time and heated to boiling until all the solid had dissolved, 8ml were required, and
filtered hot. On cooling the toluene solution deposited crystals. These were filtered off, washed with a little toluene and dried at 50 C to remove as
much toluene as possible. Examination under the microscope revealed that there are 2 compounds present; sprays of non-fluorescent yellow needles,
almost certainly unreacted bromo-anthracene and larger, blocky, triclinic looking, lustrous brownish tablets that fluoresce bright blue that are
hopefully the dianthryl. I am going to try leaching them with various solvents to see if I can removed one of the compounds preferentially.
Today I fused the remaining portion of the mixture in a flask as before until it became almost infusible. its currently cooling down.
Does anyone have any ideas for a suitable solvent mix for TLC for these compounds?
|
|
|
Niklas
Harmless

Posts: 26
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Something like 1:24 ethyl acetate / n-hexane should work fine I guess, at least that’s what I used for checking the purity of my 9-bromoanthracene
|
|
|
Niklas
Harmless

Posts: 26
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Quote: Originally posted by Boffis  | | Examination under the microscope revealed that there are 2 compounds present; sprays of non-fluorescent yellow needles, almost certainly unreacted
bromo-anthracene and larger, blocky, triclinic looking, lustrous brownish tablets that fluoresce bright blue that are hopefully the dianthryl.
|
Shouldn’t 9-bromoanthracene be fluorescent too tho?

|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Mmm yes 9-bromoanthracene is fluorescent too. Bugger. Not as fluorescent as the dianthryl and its more soluble in isopropanol than the dianthryl.
I worked up the larger scale experiment today, just waiting for the toluene extract to crystallise.
|
|
|
Niklas
Harmless

Posts: 26
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Quote: Originally posted by Boffis  | | Examination under the microscope revealed that there are 2 compounds present; sprays of non-fluorescent yellow needles, almost certainly unreacted
bromo-anthracene and larger, blocky, triclinic looking, lustrous brownish tablets that fluoresce bright blue that are hopefully the dianthryl.
|
On the topic of it, looking at my 9-bromoanthracene (made by the bromination of anthracene with NBS in chloroform) under the microscope I‘m noticing
that there is two kinds of crystals in there too, both kinda fitting your descriptions. Oddly in my case it’s the dark crystals that aren’t
fluorescent tho.. Any idea what they may be?

|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
This is very interesting, because I did not meet such Ullmann reaction, including this reactant. I would try more "standard" reaction conditions, I
mean with DMF as reaction medium (hard to call it a solvent). It lowers reaction temperature, however it gives no guarantee of succsess 
You may read a book "Synthesis of Biaryls" (I.Cepanec).
The only method for purification of this di(bi)anthryl I found is repeated crystallization from toluene (it forms molecular adduct 1:1 with it - just
a remark to remember).
BTW.
I have ~20 g of nothing-to-do anthracene and it also gives blue fluorescence under UV, as I checked long time ago.
[Edited on 13-12-2023 by kmno4]
Слава Україні !
Героям слава !
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@kmno4, do you know how stable the dianthryl-toluene adduct is? I weighed the product from my experiment mentioned above and then heated it to 70 C
for 14 hours but it lost virtually no weight at all (< 1%).
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I do not know, but such adducts are formed by weak forces and cannot be very stable. I think that toluene is removed below 100 C. If your product has
no odour of PhMe, it is probably freed of it.
I obtained similar, crystalline adduct (unknown composition) by the way of modafinil preparation. Just crystals filteres off were very strongly
toluene smelling and after standing at r.t. toluene disappeared and the crystals turn to white powder.
Info about bianthryl-toluene (and pyridine too) adduct is taken from the paper I attached (Experimental, a))
PS. during writing this post I was googling (1 minute), and TG of 9.9' bianthryl-toluene is found :
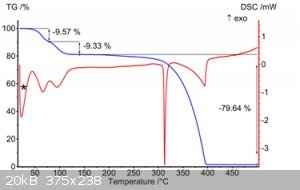
[Edited on 20-12-2023 by kmno4]
Слава Україні !
Героям слава !
|
|
|