| Pages:
1
2 |
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Preparation of Hydrogen Cyanide
Hydrogen cyanide is extremely toxic. Breathing even small amounts can kill you. The preparation of HCN must be done in a
good fume hood or in a very well ventilated area outside. Make sure that the wind never blows any vapours into your face. Prussic acid is not suitable
as a homicidal poison. It's super easy to detect and you're immediately convicted as the killer. Please don't poison yourself with it either. Death
can be very painful. Nobody wants to die like that. Please: search for professional help, if you think about killing yourself.
Procedure: I added 300 grams of 50 % sulfuric acid into a 1 liter erlenmeyer flask with stir bar. A Liebig condenser and a receiving
flask (ice cooled) were attached. Under fast stirring, I added 250 grams of powdered potassium ferrocyanide trihydrate.

A bluish white slurry formed.

I switched on the heating and after some minutes of stirring the first liquid condensed, but it wasn't very warm. The liquid is a mixture of hydrogen
cyanide with a bit of water. When less hydrogen cyanide condensed, the temperature was raised from time to time in small steps. When the drops didn't
wet the glass anymore, the majority of the hydrogen cyanide is driven off and the condensing liquid consists mainly of water.

At this point, stirring and heating were switched off. I received about 80 milliliters of HCN, contaminated with traces of sulfuric acid and some
water.

The residue in the erlenmeyer flask is a yellowish slurry with a blue surface, containing half of the cyanide.

This can be recycled (explained further below). The impure hydrogen cyanide had to be dried. I combined two batches of HCN (from this 250 g
ferrocyanide + from another 80 g). I used anhydrous calcium chloride, made by heating CaCl2 dihydrate. CaCl2 was added in small
portions and the flask was swirled around. At the bottom, a layer of aqueous saturated CaCl2 solution formed and the supernant became
cloudy.

I added more CaCl2 until the supernant was clear again, and sucked up the CaCl2 solution with a pipette. This was discarded. To
stabilize the anhydrous HCN, one can use a tiny amount of 85 % phosphoric acid. I added a small drop (~30 mg) into a washing bottle.

The HCN was again distilled directly from the flask, still containing the calcium chloride, into the ice-cooled receiving flask (washing bottle). I
also added some peaces of anhydrous calcium chloride into the condenser. The HCN boiled at about 25 °C (in a water bath).

The temperature was rised slowly. When no more liquid condensed, the operation was done (at about 40-50 °C of the water bath). The residue was still
wet and contained some hydrogen cyanide.

Weight of the anhydrous HCN from 330 grams of ferrocyanide: 58 grams.
Yield: 92 % (based on the reaction scheme)
The density was measured with a volumetrical flask and an electronic scale.
Measured density: 0.6924 g/ml
Theoretical density: 0.6876 g/ml
I filled all of the hydrogen cyanide in ampoules and sealed them with a bunsen burner.

After more than one year, the HCN in all of them is still crystal clear. I also made some unstabilised HCN (without phosphoric acid). After one day,
they looked yellowish (left ampoule):

After one year, the unstabilised HCN formed a brown solid (left ampoule).


So even 0.05 % of phosphoric acid prevents this polymerisation reaction for more than one year (according to the literature: even much longer).
Recycling of the side product: The residue from the preparation is so called Everitt's Salt. To convert this back to ferrocyanide,
the erlenmeyer flask was left open, so that the salt could be oxidised by air. I occasionally swirled the flask. When the whole mixture was dark blue,
the oxidation was complete.

I filtered and dried the blue compound.

The filtrate was neutralised with some soda and flushed down the drain. The blue compound was added to a soda solution, containing about 45 grams of
soda in 600 ml water.

Under stirring, the whole mixture was heated and more soda added in small portions until the colour changed to brown.

While still warm, I filtered it.

The filtrate was evaporated somewhat, cooled, and the yellow crystals of ferrocyanide collected.

The rest of the filtrate was evaporated to dryness and the residue was combined with the crystals. The stuff in the filter is iron oxide. I dried,
weighted and discarded it.

Yield: 121 grams of Na/K ferrocyanide hydrate (less than expected, I don't know why)
Weight of the iron oxide: 40 grams (more than expected, I don't know why)
I successfully used the recycled salt in another batch.
Explanation:
Potassium ferrocyanide reacts with sulfuric acid to form hydrogen cyanide, potassium hydrogen sulfate and a complex salt, called Everitt's Salt.
2 K4[Fe(CN)6] + 6 H2SO4 → 6 HCN + 6 KHSO4 + K2Fe[Fe(CN)6]
As you can see, only half of the cyanide is converted to hydrogen cyanide. With this method, that's the maximum yield. A patent claimes that you can
get 100 % yield by using copper chloride as a catalyst.
The Everitt's Salt can be recycled in two steps. First the oxidation by air:
4 K2Fe[Fe(CN)6] + 4 KHSO4 + O2 → 4 K[Fe2(CN)6] + 4 K2SO4 + 2
H2O
The product is called Prussian Blue. This is a pigment, from which HCN was first made in early days. That's why HCN is also called "Prussic Acid".
To convert Prussian Blue to Ferrocyanide, it can be reacted with soda:
4 K[Fe2(CN)6] + 6 Na2CO3 → K4(Fe(CN)6) + 3 Na4(Fe(CN)6) + 6
CO2 + 2 Fe2O3
The product is K/Na ferrocyanide in the molar ration 1:3. If you use potash instead of soda, you will get pure potassium ferrocyanide.
Sources:
Preparation of anhydrous HCN:
Trautwein: Darstellung der trocknen Blausäure (a). In: Gmelin, L. (1848) Handbuch der organischen Chemie: Organische Chemie im Allgemeinen. - Organische
Verbindungen mit 2 und 4 Atomen Kohlenstoff. 4. Auflage, Heidelberg, Winter, p. 314.
Stabilisation of HCN with H3PO4:
Walker (1938) Stabilization of liquid hydrocyanic acid. Patent US2194370A.
Reaction schemes:
Kraft (2014). On the History of Prussian Blue: Thomas Everitt (1805-1845) and Everitts Salt. Bulletin for the history of chemistry / Division of the History of
Chemistry of the American Chemical Society. 39. p. 18-25.
Czelechowsky, J. R. (1841) Chemisches Wörterbuch zum Gebrauche Ärzte, Pharmaceuten, etc, Carl Gerold, p. 144.
Catalysed HCN production:
Dewrance & Williams (1909) Verfahren zur Darstellung von Alkalicyanid. Kaiserliche Patentschrift Nr. 224950.
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
This is just a q&d description. I'm also making a video about it with more details and better footage....

....and some experiments with HCN and so on. But I don't know, where to upload it.
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
That photo is beautiful and horrifying at the same time 
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Beautiful write-up.
Interesting to see that you can isolate such pure HCN from a an innocuous chemical like potassium ferrocyanide. Also good to know that phosphoric acid
stabilizes HCN.
Do you have plans for further interesting experiments with HCN? It sounds like a really interesting, albeit it somewhat scary, chemical. Only
something for people who really know what they are doing and know the risks of working with it.
Does it also stabilize aqueous solutions of HCN, prepared from NaCN or KCN and some acid?
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I like it very much, really. I perforformed it (many times) without HCN isolation in larger amounts, just droples of distiiled off HCN were introduced
directly into ethanolic KOH solution.
Formation of very acid-stable K2Fe[Fe(CN)6] is annoying, I also converted it back to K4[Fe(CN)6] (with KOH), but it is very messy job.
Much better solution is using small amount of CuCl "catalyst" (see 'cyanide thread'). It distroys all ferrocyanide, via CuCN formation and
destruction*.
| Quote: | | Please: search for professional help, if you think about killing yourself |
It sounds very, very oddly       
*E.Williams, J. of the Soc. of Chem. Ind., 1912, pages 468-471
[Edited on 23-1-2024 by kmno4]
Слава Україні !
Героям слава !
|
|
|
unionised
International Hazard
    
Posts: 5104
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Everyone knows that HCN is very toxic.
Not many people realise it's explosive.
https://www.icheme.org/media/8681/paper-52-hazards-25.pdf
Sealing explosive, toxic chemicals in glass ampoules is... not recommended.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Very interesting! I am looking forward to that video.
I found years ago very interesting document about chemistry of liquid HCN and saved it. Look at it, maybe you find some inspiration for interesting
experiments with HCN.
Wow, I haven't know that hydrogen cyanide can explode on its own!
[Edited on 23-1-2024 by Bedlasky]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Explosive polymerisation is possible, but very rare in small amounts, I think. It's true that my two unstabilised ampoules could have exploded. But if
you stabilise the HCN the way I described it, no explosions can occur.
Quote: Originally posted by woelen  | | Do you have plans for further interesting experiments with HCN? It sounds like a really interesting, albeit it somewhat scary, chemical. Only
something for people who really know what they are doing and know the risks of working with it. |
I initially made it as reagent to make a complex compound and to make pure KCN for other experiments. But I just made much more than needed to examine
some of its properties. I will show some HCN properties in this thread.
Yes, it's really dangerous stuff, especially if you belong to the people who can't smell it. The smell (rather a stink) is the last warning sign, in
my opinion. Two or three times I smelled more than a faint fragrance and I quickly felt dizzy.
I don't know, but aqueous solutions are said to be stabilised by many acids at least for some time. Before drying the crude HCN (contaminated with
only a tiny bit of sulfuric acid and a few percent of water) I stored it in the freezer for nearly two weeks and it still looked perfectly clear. But
as soon as it was free of water and free of traces of acid, it turned yellow within one day even in the freezer. According to the patent, phosphoric
acid is by far the best stabiliser. I could imagine that this is also true for aqueous HCN, if it's very dilute.
I did the preparation in 2020 and checked the stabilised ampoules just a few weeks ago. They all still look perfect. Although the stabilised product
doesn't seem to be light-sensitive, I store it in complete darkness. I think it will be unaltered for some decades.
Quote: Originally posted by kmno4  | | Much better solution is using small amount of CuCl "catalyst" (see 'cyanide thread'). It distroys all ferrocyanide, via CuCN formation and
destruction*. |
Yes, I also read this after performing the experiments. Sounds much better, though I'm a bit sceptical about the chloride. Doesn't it form HCl? I'm
afraid it could contaminate the product. Maybe one can use copper sulfate to avoid this.
Quote: Originally posted by kmno4  | | Quote: | | Please: search for professional help, if you think about killing yourself |
It sounds very, very oddly        |
Unfortunately, many people search for this compound for this purpose. There is a certain rule for journalists that they at least add this helping
info, to stop people from doing that. So I just also did it.
[Edited on 23-1-2024 by Pok]
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Very nice work! I love these ampuoles 
It is interesting to see that adenine is formed after polymerazion of HCN
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Yeah, I found that interesting too! Good idea for some sci-fi or fantasy story on the planet with HCN oceans and HCN based life  . .
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Properties of Hydrogen Cyanide
HCN has a low viscosity. When you shake it, small "waves" can be seen:

When frozen in a fridge, it solidifies to an ice-like solid:

During melting, the solid sinks to the buttom (unlike ice in water):

It's very volatile. Boiling point: ~ 26 °C. When some of it is poured on a warm surface, it vaporises rapidly:
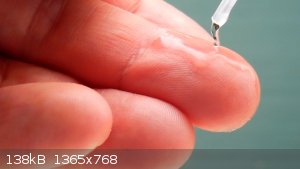
Note: this should not be repeated. My hand was warm and dry. I blew hard to let the HCN evaporate within 3-5 seconds. Under other circumstances, this
could be dangerous.
Some old textbooks say, that hydrogen cyanide can partially freeze during evaporation due to the cooling effect. I slowly let flow some of it out of a
pipette on a cold day (+ 10 °C) with high air humidity. An "icicle" formed. I doubt that this is solid HCN. I rather think it's frozen water vapour.
On the other hand, the water should dissolve in the liquid HCN, so this is a bit strange:


HCN is said to burn with a bluish flame. Unfortunately, this wasn't the case in my experiment. It looked like burning ethanol:

HCN is a very weak acid. It doesn't react with soda:

From left to right: dilute H2SO4 (reaction already complete), dilute acetic acid, pure HCN
The anhydrous liquid doesn't even react with magnesium powder:

From left to right: dilute H2SO4 (reaction already complete), dilute acetic acid, pure HCN
But if some water is added, it reacts violently with it (right test tube):

A pH test paper confirms that HCN is a weak acid. The paper doesn't change its colour:

left: H2SO4 (10 %), middle: acetic acid (10 %), right: HCN (~ 50 %)
Nevertheless, HCN is of course dangerous due to it's toxicity. I caught a mosquito* and tested this. After 10 seconds, it was dead:



*I love animals, but I would have killed this mosquito anyway. So I find it ethically legitimate so sacrifice it for this experiment.
Further experiments (in planning):
- qualitative HCN test (paper)
- pur HCN into ethanolic KOH solution to show the formation of a salt
When this is done, I can finish the video and upload it somewhere.
|
|
|
Belowzero
Hazard to Others
  
Posts: 173
Registered: 6-5-2020
Location: Member Is Offline
Member Is Offline
|
|
Very nice, thank you for sharing this with us.
Perhaps you can consider writing a report for the prepublication section.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
That demo with your finger,  , I love it. , I love it.
Lmao
[Edited on 24-1-2024 by Tsjerk]
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by Bedlasky  | Very interesting! I am looking forward to that video.
I found years ago very interesting document about chemistry of liquid HCN and saved it. Look at it, maybe you find some inspiration for interesting
experiments with HCN.
[Edited on 23-1-2024 by Bedlasky] |
Sorry, I noticed that I didn't post link to that paper  . Here it is: . Here it is:
https://sci-hub.se/https://www.sciencedirect.com/science/art...
There are mentioned some colorful transition metal complexes of HCN in table 5 and 6.
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Great! Keep up good work. Also perfect experiment with the mosquito, so everyone could imagine how quickly the HCN causes its lethal effect. There was
a forum in my country, but is already dead. 20 y ago a member with nick Cyano posted there his experiments with HCN distillation in his cellar and
that all the rats there died (luckily he survived). Antidotes are inhaling amyl nitrite + O2, intravenous infusion of NaNO2 and Na2S2O3, vitamin B12
and many more.
edit: I found his post in an archive in my harddisk, I like to save interesting things as they can disappear in the future, the forum had a name PXD:
| Quote: | od: cyano 01.07.2003, 02:44:32
Též jsem dělal kapalný kyanovodík-- ale podcenil jsem délku chladiče a teplotu chladicí kapaliny-- byla 17 st, je zde nutná teplota kolem
nuly. Došlo k explozi aparatury--nic se nestalo, pokus jsem dělěl ve sklepě rod. domku-- ale vše je k něčemu dobré-- pochcípali
potkani--našl jsem jich další den asi 5-7 , nevím , bylo to před lety, když jsem chodil na gympl.
here the translation into eng:
I also did liquid hydrogen cyanide - but I underestimated the length of the condenser and the temperature of the cooling liquid - it was 17 C, the
required temperature is around zero. There was an explosion of the apparatus - nothing happened, I was doing the experiment in the basement of the
family house - but everything is good for something - the rats perished - I found about 5-7 of them the next day, I don't know, it was years ago when
I studied Gymnasium (Gymnazium in my country is analogy of high school or grammar school). |
[Edited on 25-1-2024 by Fery]
|
|
|
Admagistr
Hazard to Others
  
Posts: 350
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
@Fery: You did some rough experiments when you were in high school! I remember one of our Forum members, he was a tenager, he was not even 15 years
old, died trying to make HCN! I think his nickname was Myfanvy2013, or something like that...Glad you're alive
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Beautiful and very interesting set of experiments with the HCN. The experiment with the liquid evaporating on your finger must be quite thrilling  . I would be quite nervous about this. A dose of 50 mg can kill an adult healthy
person! . I would be quite nervous about this. A dose of 50 mg can kill an adult healthy
person!
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 163
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
Excellent write-up: you're extremely brave for carrying out this work (much more so than I am), and you clearly know what you're doing.
On a similar subject, I have come across a claim on the wiki that calcining Prussian Blue with molten caustic soda produces sodium cyanide directly,
without forming any intermediate HCN. It would be a very useful method if true, but I've not been able to find any supporting sources, probably
because my search-fu game is weak.
[Edited on 25-1-2024 by Lionel Spanner]
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Quote: Originally posted by Admagistr  | @Fery: You did some rough experiments when you were in high school! I remember one of our Forum members, he was a tenager, he was not even 15 years
old, died trying to make HCN! I think his nickname was Myfanvy2013, or something like that...Glad you're alive |
The young last classes of elementary school aged guy who died used the nick Virius, he made a lot of acetone peroxide and we knew only thanks to his
friend with the nick mistr.vodic who wrote us what happened (mistr.vodic was only the tester of the product and the poor Virius only the producer).
It happened in February 2006.
[Edited on 25-1-2024 by Fery]
|
|
|
Admagistr
Hazard to Others
  
Posts: 350
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by Fery  | Quote: Originally posted by Admagistr  | @Fery: You did some rough experiments when you were in high school! I remember one of our Forum members, he was a tenager, he was not even 15 years
old, died trying to make HCN! I think his nickname was Myfanvy2013, or something like that...Glad you're alive |
The young last classes of elementary school aged guy who died used the nick Virius, he made a lot of acetone peroxide and we knew only thanks to his
friend with the nick mistr.vodic who wrote us what happened (mistr.vodic was only the tester of the product and the poor Virius only the producer).
It happened in February 2006.
[Edited on 25-1-2024 by Fery] |
I found it: https://www.sciencemadness.org/whisper/viewthread.php?tid=13...
In another thread, which I can't find right now, he is listed in our list of members who died in their experiments. It said he was doing experiments
with HCN. His nickname was myfanwy94 and he was about 15 years old.
[Edited on 25-1-2024 by Admagistr]
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Thx for the link. Be all safe and enjoy chemistry. This is apparently another guy as he was posting in 2010. The guy I mentioned was a member of PXD
or later cz pyroforum and that disaster happened in Feb 2006. He was posting in cz language in one of those forums (his friend or maybe schoolmate
too), not here in sciencemadness.
Back to topic, it's heartwarming to see skilled forumers here and the beautiful experiments and pictures most of us won't have braveness to do. They
are inspiring all of us. The route via HCN seems to be the right way how to further produce very pure NaCN / KCN. Also very informative how to
stabilize the product for its longer storage (I didn't know about its polymerization at all). Also very useful how to take in account the economy and
recover half of the reactant to be reused in subsequent reactions.
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Nice. Cyanides are really a good starting material to do a ton of interesting chemistry.
Quote: Originally posted by Fery  | | Antidotes are inhaling amyl nitrite + O2, intravenous infusion of NaNO2 and Na2S2O3, vitamin B12 and many more. |
I didn't even know that antidotes exist. I just found out that a KGB agent killed two famous Ukranians with an HCN filled "gun" during the cold war.
One of them was Stepan Bandera. I often read his name in the media (related to the war in Ukraine). Right before the attack, the agent indeed took a
pill of sodium thiosulfate and immediately afterwards he inhaled amyl nitrite.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Stepan Bandera is a hero in Ukraine, it is true.
But in reality, he was ultra-nationalist collaborating with Nazi Germany and was responsible for ethnic cleansing. Ukrainians love him, but
unfortunately, for me he is (more or less) a bandit. By the way of Germany in II WW.
They made large use of HCN for mass murderes in their Death Camps. Of cource, it was Zyklon B, produced escpecially for this purpose by German
industry. Yes, genocide on industrial scale. It is very sad, especially when young Germans say that it was not Germans, it was some Nazists.....
It is called "relativization of history".
BTW.
Most of murderers from Death Camp were not Germans, but Austrians. Also Adolf H. was an Austrian.
Today, Austrians are very angry when somebody gives these facts and wish that all other people forget about it 
[Edited on 27-1-2024 by kmno4]
Слава Україні !
Героям слава !
|
|
|
Admagistr
Hazard to Others
  
Posts: 350
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by kmno4  |
Stepan Bandera is a hero in Ukraine, it is true.
But in reality, he was ultra-nationalist collaborating with Nazi Germany and was responsible for ethnic cleansing. Ukrainians love him, but
unfortunately, for me he is (more or less) a bandit. By the way of Germany in II WW.
They made large use of HCN for mass murderes in their Death Camps. Of cource, it was Zyklon B, produced escpecially for this purpose by German
industry. Yes, genocide on industrial scale. It is very sad, especially when young Germans say that it was not Germans, it was some Nazists.....
It is called "relativization of history".
BTW.
Most of murderers from Death Camp were not Germans, but Austrians. Also Adolf H. was an Austrian.
Today, Austrians are very angry when somebody gives these facts and wish that all other people forget about it 
I read that during WWII the Ukrainians initially welcomed the Germans with the hope that they would liberate them from the Russians.Maybe the Bandera
felt the same way, that the Germans would liberate them from the Russians? Yes the HCN was massively abused to murder defenceless and innocent people
especially Jews, killing millions of them!!!
[Edited on 27-1-2024 by Admagistr] |
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Admagistr  | | I read that during WWII the Ukrainians initially welcomed the Germans with the hope that they would liberate them from the Russians.Maybe the Bandera
felt the same way, that the Germans would liberate them from the Russians? |
Yeah, a lot of Ukrainians considered the Nazis to be the lesser of two evils- the Nazis talked about killing millions of Jews, but the Stalinists had
already killed ten million Ukrainians. Tough to blame them.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
2 |