Synthesis of 1-amino-2-naphthol
Hello people !
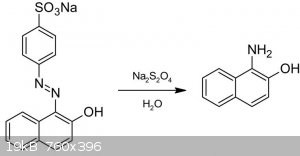
Today next episode of old classic series: the reduction of Orange II to 1-amino-2-naphthol. The reaction is a reduction of the dye in an aqueous solution of sodium dithionite. Very easy workup
and purification.
Procedure
The dye Orange II prepared last time and left in the paper filter, is introduced in a large beacher, added of 800 ml of water and brought to 85 Celsius on hotplate under
stirring. A deep red solution is obtained. To this solution, 36 g of sodium dithionite is added in portion during which, red color turn to orange and
then to a final yellow. A gentle froating occurs, and a precipitate forms. The yellow suspension is kept at 85 Celsius under stirring until volume
goes down to 400 ml. This is vacuum filtered still hot and washed thouroughly with water, pump dried for a while, then pressed on paper. The residue
quickly darkens in air to an olive-brown powder, 7.12 g ( 79.11% considering Orange II as 100% last reaction step ).
m.p.: 195 - 198 Celsius

Discussion
Aromatic amines are known to degrade in air, aminonaphthol is not an exception, in fact I stored at -18 Celsius.. Next step we oxidize this to
naphthoquinone, and the old classic series will be end.
References
Org. Syn. vol. 17, pag. 9, 1937.
As usual, I thank you for attention, and link to YT video for a more detailed procedure.
See you,
palico
[Edited on 16-2-2024 by palico]
|