ZHANGNIUBI
Harmless

Posts: 28
Registered: 5-5-2011
Member Is Offline
Mood: No Mood
|
|
need help of a reaction's mechanism
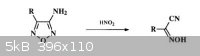
It‘ s about aminofurazan's diazotization(with HNO2--mix of NaNO2/HOAc). when the reaction occurs, a-Hydroximinoacetonitriles were common products.
The problem is how can a C+ becomes a C- . My point is by oxizdation of HNO2, but it seems to be weired. At least, I ve never seen a reaction like
that before. My friend thought that it may form a structure like C-N=N-O- and lose N2O to form a C-, but from my point of view, it's wrong again,
'cause usually, a C-N=N-O- is obtained in strong base like conc.KOH.
Does any body know the answer? Thank you!
|
|
|
ZHANGNIUBI
Harmless

Posts: 28
Registered: 5-5-2011
Member Is Offline
Mood: No Mood
|
|
。。。
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
This reaction is not known in the literature, or at least not the way you've drawn it. I would not recommend you invoke anions under acidic
conditions, this is a fairly universal rule. So, I disagree with your friend as well.
As you pointed out, the starting material is in a higher oxidation state than the product, and thus must undergo a reduction. A good idea when
drawing mechanisms is to consider the oxidation state of starting materials and products to determine whether or not it is a redox reaction (see
scheme A).
- rings = +1
- heteroatoms = +1
- pi bonds = +1
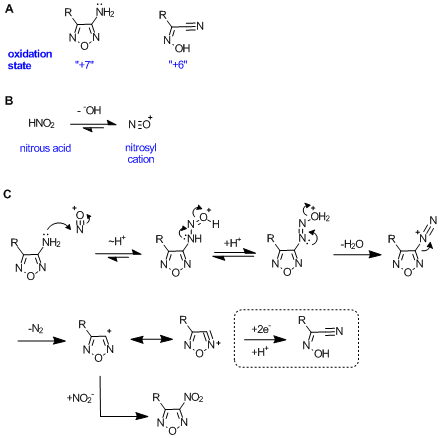
So others know what you're talking about, nitrous acid is formed, and dissociates into the nitrosonium cation (scheme B). This would
lead to diazotization by a mechanism such as shown in C, but cannot proceed to product due to lack of a reducing agent. According to
literature precedent, this diazonium salt leads to a nitrofurazan in the presence of sodium nitrite. Obviously it could add any nucleophile present.
If any one of these intermediates were reduced, monosubsitututed 1,2,5-oxadiazoles decompose spontaneously, or under acid/basic conditions to give
alpha-oximinonitriles by cycloreversion - you know this it seems.
Other options might include radical fragmentation or intramolecular hydride delivery, but I'm not sure exactly how.
[Edited on 7-1-2012 by Arrhenius]
|
|
|
ZHANGNIUBI
Harmless

Posts: 28
Registered: 5-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | This reaction is not known in the literature, or at least not the way you've drawn it. I would not recommend you invoke anions under acidic
conditions, this is a fairly universal rule. So, I disagree with your friend as well.
As you pointed out, the starting material is in a higher oxidation state than the product, and thus must undergo a reduction. A good idea when
drawing mechanisms is to consider the oxidation state of starting materials and products to determine whether or not it is a redox reaction (see
scheme A).
- rings = +1
- heteroatoms = +1
- pi bonds = +1
So others know what you're talking about, nitrous acid is formed, and dissociates into the nitrosonium cation (scheme B). This would
lead to diazotization by a mechanism such as shown in C, but cannot proceed to product due to lack of a reducing agent. According to
literature precedent, this diazonium salt leads to a nitrofurazan in the presence of sodium nitrite. Obviously it could add any nucleophile present.
If any one of these intermediates were reduced, monosubsitututed 1,2,5-oxadiazoles decompose spontaneously, or under acid/basic conditions to give
alpha-oximinonitriles by cycloreversion - you know this it seems.
Other options might include radical fragmentation or intramolecular hydride delivery, but I'm not sure exactly how.
[Edited on 7-1-2012 by Arrhenius] |
well, this reaction actually was found on :
http://www.docin.com/p-43518848.html?bsh_platform=renren
on the page 131, scheme 130. the article part shows some papers(index), I m not sure if those can help. because I can't read them...maybe you can use
your school's online library to figure it out. and I ll be very grateful if you can share some of them
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Okay, thanks. I found it, but I can only get a few of the papers cited - most are foreign journals, and I don't read Russian anyway. Of the one I do
get, and ran into before I posted, they claim to obtain the oximinonitrile byproduct when attempting a Sandmeyer reaction. They used 1eq of copper 1
or 0, and got poor yield of methyl oximinonpropionitrile. Copper is serving as the reducing agent. They draw both the radical (as I've shown) and a
cation, which is bloody confusing, because I thought they meant radical cation for a moment, but it could be the cation as well, which ultimately must
undergo one electron reduction and hydrogen abstraction (e.g. from solvent) or a second one electron reduction and a proton abstraction. Maybe you
could get a good yield with the proper stoichiometry of copper - i.e. >2eq.
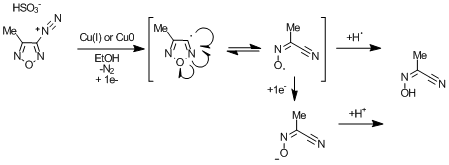
Rakitin, O.A. et al, Russian Chem. Bull., (1993) 42(11) 1865.
Oy. Here's another idea from a paper that yields a similar product (also cited in the book). Not sure I buy this one, but you decide. I don't even
know what to call NO2+  . .
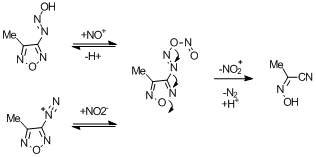
Churakov, A.M. et al, Chem Heterocycl Comp. (1988)24, 1378.
[Edited on 7-1-2012 by Arrhenius]
|
|
|
ZHANGNIUBI
Harmless

Posts: 28
Registered: 5-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | Okay, thanks. I found it, but I can only get a few of the papers cited - most are foreign journals, and I don't read Russian anyway. Of the one I do
get, and ran into before I posted, they claim to obtain the oximinonitrile byproduct when attempting a Sandmeyer reaction. They used 1eq of copper 1
or 0, and got poor yield of methyl oximinonpropionitrile. Copper is serving as the reducing agent. They draw both the radical (as I've shown) and a
cation, which is bloody confusing, because I thought they meant radical cation for a moment, but it could be the cation as well, which ultimately must
undergo one electron reduction and hydrogen abstraction (e.g. from solvent) or a second one electron reduction and a proton abstraction. Maybe you
could get a good yield with the proper stoichiometry of copper - i.e. >2eq.
Rakitin, O.A. et al, Russian Chem. Bull., (1993) 42(11) 1865.
Oy. Here's another idea from a paper that yields a similar product (also cited in the book). Not sure I buy this one, but you decide. I don't even
know what to call NO2+  . .
Churakov, A.M. et al, Chem Heterocycl Comp. (1988)24, 1378.
[Edited on 7-1-2012 by Arrhenius] |
Wow, the second one seems to be what I need..
thank you very much!!
|
|
|
|