freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
Distillation of H2SO4 > What tubing stands up to it?
Is there any other types of tubing except glass that can withstand high concentration H2SO4? Aluminum works for a bit with HNO3. PTFE (Teflon) works
nicely for HNO3 too.
I am highly skeptical of adding a few percent of 30% H2O2 to lower quality H2SO4 to help purify it before heating. It appeared to boil off on the
surface. After bringing the H2SO4 to boiling it was cooled and was a very clear slightly yellow liquid. Not sure if the H2O2 made any difference
because the acid was already pretty much clear.
[Edited on 27-1-2012 by freedompyro]
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
I would think that simple polyethylene would work, unless it is very hot acid, which will melt the tubings. SO3, on the other hand, must require
PTFE/glass tubings.
|
|
|
per.y.ohlin
Harmless

Posts: 27
Registered: 14-11-2009
Member Is Offline
Mood: No Mood
|
|
If you are dealing with simply boiling down the acid, then I don't see the point of tubing (collecting and neutralizing the mist maybe?). If you are
actually evaporating and condensing H2SO4 in another flask, then it boils at 338ºC [1]. Polyethylene melts between 100ºC and 120ºC, so that won't
work [2]. If you end up doing this, I doubt any plastics will handle the temperature, let alone the corrosive acid. Ground glass might work. Either
way I strongly advise against this method. This is a lot hotter than most experiments get, and there is a risk of failing to collect the acid mist, or
destroying your glassware and spilling the concentrated acid everywhere.
[1] http://www.qvf.com/en/processsystems_3/mineral%20acids/Conce...
[2] http://wiki.answers.com/Q/What_is_the_melting_temperature_of...
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Distilling H2SO4 in a home/amateur setting does not sound like something I would do. I would strongly advice against it, because of the high
temperatures involved. Distilling fuming HNO3 (which certainly is as corrosive as concentrated H2SO4) can be done at home in good glassware, because
of the much lower boiling point. I would be realy scared if there were a distillation setup, heated up to well over 300 C filled with fumes of H2SO4.
A better method might be to try to find a source of battery acid, which is much clearer than most drain cleaners. The battery acid can be boiled down
to 90+ % and you obtain a nearly colorless (slightly brown) acid.
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
Meh... I'm just being obsessive-compulsive in wanting perfect reagents with no trace contaminants... My H2SO4 works perfect and appears the way
technical grade should even though it's from a drain cleaner. This is it's color after being brought to boiling.
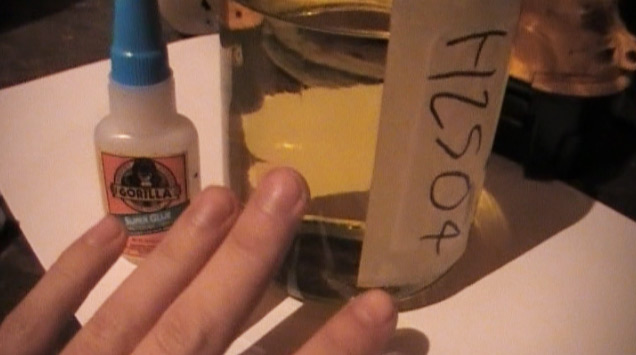

[Edited on 27-1-2012 by freedompyro]
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
As long as the colouring doesn't inhibit any reactions, it should be fine. There is no need to obtain a perfectly clear acid.
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
Pure 98% H2SO4 should be a slight yellow color according to Wikipedia. Btw, should I have left it slightly boiling for a while, or is just bringing it
to boiling fine?
Edit > Btw... That light is terrible. With only one inch poured into that bottle it appears white.
[Edited on 27-1-2012 by freedompyro]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
No, pure 98% acid is not slight yellow. It is clear like water and absolutely colorless. For most reactions though, it is not necessary to have such
colorless acid. The wikipedia page does not tell that it should be slight yellow, it tells that in practice it is colorless to slight yellow, but if
it is slight yellow, then it is somewhat contaminated. For practical purposes this usually is not an issue.
The picture on the Wiki page is reagent grade H2SO4 in a clear glass bottle and this liquid is totally colorless. The pale yellow/green color is due
to the color of the glass, not of the acid inside the glass bottle.
I myself have some reagent grade H2SO4 (actually, the bottle on Wikipedia is a picture from mine  ), but for 99% of all my experiments I use other acid, which has a color, close to what you show on your picture. ), but for 99% of all my experiments I use other acid, which has a color, close to what you show on your picture.
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
What contaminants in general cause the yellow coloration? I guess distillation is the only way to get perfectly clear H2SO4... Need some good
glassware for that. 
Doing it with a vacuum to lower the temps might make it safer. I'm not so sure a water jacketed condenser wouldn't shatter. A air condenser would
probably be best.
[Edited on 27-1-2012 by freedompyro]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I was told that the most occurring contamination of sulphuric acid is small amounts of (partially charred) organic materials and also possibly some
transition metal compounds (the latter in minute quantities). For this reason, people often suggest using H2O2 and boil off this H2O2. While doing so,
the H2O2 oxidizes all organic matter to CO2, H2O and if there is S and N to SO2 and NOx. All of these can be boiled away and the acid becomes much
clearer. With H2O2 you do not remove inorganic impurities like transition metal salts.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Don't want to insult anyone, but let me just burst a few bubbles by saying that I wouldn't recommend even less dangerous experiments to someone that
keeps his concentrated sulphuric acid in a food product jar (yet complains about trace contaminants) and doesn't care labeling it H<sub>2</sub>SO<sub>4</sub> instead of H2SO4.
Sulphuric acid would surely start to decompose upon boiling, probably creating a mess in the condenser. It's one of the toughest acids to boil, a
property sometimes quite useful, and sometimes not.
I have an all glass distilling apparatus and quite a bit of experience in the lab, but I wouldn't use it for doing this.
[Edited on 27-1-2012 by Endimion17]
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
| Quote: | | Is there any other types of tubing except glass that can withstand high concentration H2SO4? |
Stainless steel piping is used in industry (the grade escapes me now) Mild steel was used fairly extensively at one time but corrosion problems were
problematic!
Very hot, concentrated H<sub>2</sub>SO<sub>4</sub> is a very aggressive beast ─ as I think we all know!
P
|
|
|