freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
Direct WFNA distillation without vacuum
Just a theory of mine that I have been wanting to test for a long time. Anyone got any thoughts on this?
I might be a misinterpreting the process of how pure oxygen drives NOX out of RFNA.
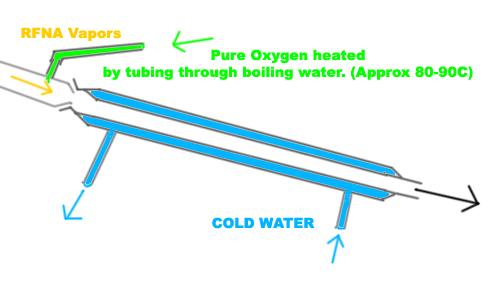
[Edited on 27-1-2012 by freedompyro]
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Seems possible, but the RFNA must contain enough water for NO2 to react. Also the temperature should be kept low, because HNO3 tends to decompose.
And I think fresh air would do the work, no need for pure O2 
Rest In Pieces!
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
Also, contrary to what people say about doing distillations slowly for low NOX... I find the clearest acid without a vacuum setup is made by putting
as many BTU's as practically possible without boilover/splatter into the bottom of a flask 75% full. To prevent bubble-over a nitrate/acid ratio of
1/1.6 and higher is needed. I then push it into a Liebig condenser running 1C ice water. I use a high speed fountain pump in a bucket with half ice
and half water. As the water comes out of the Liebig it is run on top of the ice, not under.
The condenser will not have any visible NOX discoloration from around 5% of the way into the distillation to around 70%. My theory is that to have the
least HNO3 breakdown it's best to get it into the condenser as fast as possible so that it spends the least amount of time possible as a vapor.
Putting a lot more heat into the bottom of the flask isn't going to change the distillation temperature very much as the dominant factor determining
boiling point is the ratio of HNO3/H2O to H2SO4.
The first 5-40% of the acid that distills off works excellent for Hexamine nitration after it's been cleared up.
At the start NOX is visible through the entire apparatus. Once you pick up speed the condenser is white all the way through with no visible NOX at any
point from evaporation to condensation. This is just starting to pick up speed:

Towards the end of the nitration NOX becomes very visible as shown below.

Excuse the redneck adapter.  But, it actually works better than a glass adapter
as a glass adapter makes the HNO3 vapor spend more time as a hot vapor. But, it actually works better than a glass adapter
as a glass adapter makes the HNO3 vapor spend more time as a hot vapor.
It appears that NOX is only formed at the surface of a solution of KNO3/H2SO4 and in hot HNO3 vapor. However, I could be wrong and merely bringing the
solution to boiling is generating NOX inside the solution itself.
[Edited on 27-1-2012 by freedompyro]
|
|
|