mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
Generating hypochlorite via electrolysis
I tried making chloroform from bleach and found out how horrible inefficient the whole process was. For 1L of bleach, I got 20mL of CCl3. It wouldn't
be a problem if I had mass scale glassware but the biggest thing I have is a 1000mL conical flask which I never really use. I need a better source of
hypochlorite (I don't want to order it from pool supply shops) so I'm thinking of making an electrolytic cell to convert NaCl into NaClO. Can I keep
producing NaClO until the solution becomes saturated with it?
[Edited on 21-3-2012 by mycotheologist]
|
|
|
inspector071
Hazard to Self
 
Posts: 69
Registered: 20-2-2012
Member Is Offline
Mood: No Mood
|
|
Why wouldn't you want to get it from a pool supply shop? I got a 1lb bag of the stuff for somewhere around $5. Why go to the trouble of making an
electrolytic cell to make it when it can be acquired inexpensively?
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
What type of power supply will you use for the electrolysis (please don't say a cell phone charger)? NaClO is easy to make if the contaminants aren't
a problem (shouldn't be problem for making chloroform - distilled).
Tank
Chemical CURIOSITY KILLED THE CATalyst.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Already a thread, and it even has real references and everything. Ahh the good old days...
http://www.sciencemadness.org/talk/viewthread.php?tid=1646
|
|
|
mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by inspector071  | | Why wouldn't you want to get it from a pool supply shop? I got a 1lb bag of the stuff for somewhere around $5. Why go to the trouble of making an
electrolytic cell to make it when it can be acquired inexpensively? |
I like the idea of making it myself. In the future I'll just buy it but for now I want to learn how to make it myself with an electrolytic cell.
Quote: Originally posted by m1tanker78  | What type of power supply will you use for the electrolysis (please don't say a cell phone charger)? NaClO is easy to make if the contaminants aren't
a problem (shouldn't be problem for making chloroform - distilled).
Tank |
A 12V power supply. I bought it to power my fume hood fan years ago but I realised I needed a more powerful one for that. Is 12V too much? I think I
have a few electronic gadgets to drop the voltage a bit. A light bulb would look good on my electrolytic cell lol.
One thing I'm wondering is what happens if I seal the electrolytic cell so that the Cl2 can't escape? Will the Cl2 redissolve in the water and
ultimately, react with all NaOH that gets formed? Or will the cell explode?
[Edited on 21-3-2012 by mycotheologist]
[Edited on 21-3-2012 by mycotheologist]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Sealing an electrolytic cell is almost always a terrible idea.
|
|
|
mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
Looks like I need more voltage. When I touched the graphite rods with the terminals of my multimeter, the voltage had gone down to 1V. I used some
pieces of silver wire instead but the reaction is incredibly slow. After about 30 minutes there was only a faint smell of chlorine/hypochlorite from
the solution. Couldn't smell any chlorine escaping from the jar.
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Are you sure your multimeter is set to measure DC volts? A light bulb in series will limit the current.
I don't mean to discourage you but if I were you, I'd just buy the calcium hypo and be done with it. If you insist on conducting electrolysis to make
sodium hypo, use graphite electrodes and run your solution through fine frit to filter out much of the graphite.
The voltage isn't that important. The current flow will dictate how much work is performed. The voltage needs to be sufficient to maintain an adequate
current flow. 12V should be plenty good.
Tank
Chemical CURIOSITY KILLED THE CATalyst.
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
I would buy a car battery charger. Mine does 13 V.
If you can go higher, go ahead. I think I've seen someone doing 48 V.
You will also need a good supply of graphite rods since they disintegrate more or less quickly.
|
|
|
manmetbril
Harmless

Posts: 5
Registered: 4-4-2012
Member Is Offline
Mood: No Mood
|
|
Look on ebay for MMO anode.
Much better than graphite, will last for ever and no contamination.
Best for cathode is Ti but stainless steel will also work when the power is on.
I use the 5.2V when using MMO anodes for NaClO3.
I think you have to keep the temp low.. maybe even under 30C. So maybe for hypochlorite even use the 3.2V.
But I have no clue how to avoid the forming of NaClO3 (it will definitely form when the temp gets high) or how much NaCl to start with.
[Edited on 4-4-2012 by manmetbril]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Making hypochlorite through electrolysis is very hard. Contamination with chlorate is very hard to avoid, even when the cell is kept cold. The reason
for this is that besides the disproportionation of hypochlorite to chloride and chlorate there also is an oxidizing reaction at the anode, where
hypochlorite+water is oxidized to chlorate. In a chlorate cell this reaction is somewhat undesirable (it reduces current efficiency with at most 33%).
Maybe if the cell is kept ice-cold and with very good mixing of the hydroxide from the cathode and chlorine from the anode you might get pyre
hypochlorite, but I doubt whether it is feasible to do this in a home setup.
A better way to make concentrated hypochlorite is starting from ordinary bleach, which already contains 4% or so of active chlorine. You add some NaOH
to the bleach and bubble chlorine through it. The chlorine can be made from calcium hypochlorite and HCl (or even better: TCCA and HCl).
|
|
|
mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by m1tanker78  | Are you sure your multimeter is set to measure DC volts? A light bulb in series will limit the current.
I don't mean to discourage you but if I were you, I'd just buy the calcium hypo and be done with it. If you insist on conducting electrolysis to make
sodium hypo, use graphite electrodes and run your solution through fine frit to filter out much of the graphite.
The voltage isn't that important. The current flow will dictate how much work is performed. The voltage needs to be sufficient to maintain an adequate
current flow. 12V should be plenty good.
Tank |
Yep I'm sure it was on DC. Although I'm a complete noob, electronics is another hobby of mine. Find it pretty fascinating which is why I like the idea
of making ClO- with electricity so much. Any idea why the graphite wasn't conducting very well? I stripped the two wires at the end of the power
source down and wrapped them around the graphite rods so they were definitely contacting the graphite. Should I wet the electrodes first? The water
was tap water that I saturated with NaCl. I'll try it again because these thin silver wire electrodes are useless. On top of that, one of the silver
electrodes keeps turning white which probably means silver chloride is building up on it.
I see. I was reading about dimensionally stabilised electrodes a while ago. Would it be worth investing in one (or two)? Don't know how much the
titanium rods would cost but I looked into indium chloride and ruthenium chloride and abandoned the idea of making one of these electrodes after
seeing how expensive these metals are. Indium was something ridiculous and the RuCl2 was something like €60/g. If only tiny amounts of Ru are needed
then maybe it would be viable as you could make a handful of electrodes and sell the extra ones on ebay.
Quote: Originally posted by manmetbril  | Look on ebay for MMO anode.
Much better than graphite, will last for ever and no contamination.
Best for cathode is Ti but stainless steel will also work when the power is on.
I use the 5.2V when using MMO anodes for NaClO3.
I think you have to keep the temp low.. maybe even under 30C. So maybe for hypochlorite even use the 3.2V.
But I have no clue how to avoid the forming of NaClO3 (it will definitely form when the temp gets high) or how much NaCl to start with.
|
Thanks! Would an ice bath not prevent NaClO3 from forming? I'm guessing the hypochlorite I get will be contaminated with NaOH as it is because some of
the Cl2 escapes, leaving an excess of Na+.
[Edited on 7-4-2012 by mycotheologist]
|
|
|
manmetbril
Harmless

Posts: 5
Registered: 4-4-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mycotheologist  |
Thanks! Would an ice bath not prevent NaClO3 from forming? I'm guessing the hypochlorite I get will be contaminated with NaOH as it is because some of
the Cl2 escapes, leaving an excess of Na+.
[Edited on 7-4-2012 by mycotheologist] |
To avoid escaping Cl2 add some NaOH to start with.
Hypochlorite is more stable in a basic env anyway.
By the way, I bought MMO really cheap from e-bay but dont see any now.
This MMO on ebay comes from scrap pool equipment (to make hypochlorite  ) )
Ah... question to woelen:
Would'nt it be a problem having NaClO3 in your swiming pool?
[Edited on 7-4-2012 by manmetbril]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
In swimming pools the electrolysis equipment makes chlorine at the anode and the concentration is kept very low. But most likely, some chlorate will
be formed in swimming pools, just as it is formed in electrolysis cells which are intended for making chlorate or hypochlorite. Chlorate is less
dangerous than hypochlorite, hypochlorite is MUCH more reactive and so, one can safely state that the very small amounts of chlorate, present in
swimming pool water, will not be a health risk.
|
|
|
mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
My electrolysis cell is working well now, there is a loud fizzing sound, along with loads of bubbles coming from one of the graphite electrodes and
some bubbles accumulating on the other electrode. Now I'm wondering how I can use the NaClO I made though. I have no idea how much is in there. If I
leave this thing on until all NaCl is consumed, will the NaOH and NaClO start getting electrolysed?
|
|
|
manmetbril
Harmless

Posts: 5
Registered: 4-4-2012
Member Is Offline
Mood: No Mood
|
|
Your graphite rod will never make it that far. You will quickly start making NaClO3 and then quickly have no graphite rod left 
=======================================
http://electrochem.cwru.edu/encycl/art-b01-brine.htm
And then search for 'Sodium hypochlorite manufacturing process'
As far as I understand only low concentrations can be made this way.
Attached (I hope) is an image and I wonder what will happen if the 'bridge' is from Ti and what will happen if the bridge is a 'salt' bridge with
gelatine.
I mean, what will happen with th Na+ ions on the left side and the -OH ions on the right?
With a salt bridge, will the Na+ ions travel through to the right or will the OH- ions go to the left or will it be the same as with a Ti bridge?
The idea is to produce chlorine gass and lead that through a third vessel with NaOH soln
edit 1: Bridge form Ti makes no sense now i guess. It will work as a cathode in the left bath and will try to be anode in right bath but plain Ti
anodes will not conduct any current.
edit 2: Changed the liquid in the right cell to HCl to 'catch' th Na+ ions.
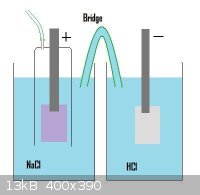
[Edited on 10-4-2012 by manmetbril]
|
|
|
mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by manmetbril  | Your graphite rod will never make it that far. You will quickly start making NaClO3 and then quickly have no graphite rod left  |
Really? Why is that? As for making NaClO3, I was wondering what happens when you perform electrolysis on a solution containing only NaClO. Is that
what happens, it converts it into NaClO3?
I've came to this conclusion myself yesterday. I had the cell running for a few hours, one of the electrodes was bubbling like mad the whole time but
after 2 hours, I smelled the solution and there was only a faint smell of bleach. Household bleach smells 20 times stronger and it only contains 5%
hypochlorite. I'm disappointing because I was hoping I had found a practical use for electrolysis. I heard of people producing NaClO3 this way though,
is this compound more viable to produce with an electrolytic cell? By viable, I mean the ability to produce reasonable amounts in reasonable times. As
opposed to my experiment which produced about a gram in 5 hours lol.
Thats a real interesting idea about the bridge, I've never thought of anything like this before. I have no idea what would happen either so I'm just
brainstorming here. So lets say the bridge was metal. The electrons could cross the bridge but ions couldn't so in the cathode compartment the
Cl- anion would be oxidised by the cathode but theres nothing to reduce the Na+. A problem I see here is that oxidising the Cl-
ions wouldn't make the overall charge any less positive because there would no longer be any counterions for the Na+ cations. Maybe that would make
the reaction unfavorable and maybe the Na+ would just oxidise any Cl2 that gets formed. Same principle applies for the anode compartment.
So with a salt bridge. The Na+ ions from the cathode compartment would instantly start to migrate to the anode compartment, while Cl- anions and
electrons from the anode compartment would be migrating in the opposite direction. Yeah, this would work wouldn't it. Anyone gonna volunteer to test
it out? It should be an easy apparatus to setup. I don't feel like doing it right now but I will later or tomorrow if nobody else does.
[Edited on 10-4-2012 by mycotheologist]
|
|
|
manmetbril
Harmless

Posts: 5
Registered: 4-4-2012
Member Is Offline
Mood: No Mood
|
|
OK,
This works I think.
I use 250 ml of 30% HCl and add that to 750ml of saturated NaCl soln. This should contain ~75 gram of Cl.
(Guess one can just use plain HCl but I started with this and it works fine). Anode is small MMO anode (1" by 2") cathode Ti, 5.2V. In the left bottle
I dissolved 64 gram of NaOH (1.6 mole) in 400ml water. Same in the right bottle.
I played around with all kinds of setups to isolate the chlorine gass from the hydrogen but failed miserably.
Now I just pass the Cl2 and the H2 through the Bottles.
The left bottle I 'harvest', the right one is to catch the left over Cl2. Now for a new batch I will move the right bottle to the left and put a fresh
NaOH solution in the right bottle.
Cheap! 1 liter of HCl is about 3 eur (300 gram Cl2)
While my pool chlorine was 17.5 eur for about 250 gram of Cl2 and you also need the HCl.
I know Cl2 and H2 will go boem when exposed to UV light.
As far as I know now the boom is not 'that' violent (like O2 and H2) I saw classroom experiments.
Question:
How stable and how 'strong' is this Cl2 H2 mixture or is this way just waiting for a little bang?

Amonia is there to detect leaks, cloth to protect from light.
[Edited on 13-4-2012 by manmetbril]
|
|
|
manmetbril
Harmless

Posts: 5
Registered: 4-4-2012
Member Is Offline
Mood: No Mood
|
|
Hmm... I think I forgot the escaping HCl from the electrolysis cell.
First bottle should have water to catch the HCl.

[Edited on 16-4-2012 by manmetbril]
|
|
|
mycotheologist
Hazard to Others
  
Posts: 154
Registered: 16-3-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  |
A better way to make concentrated hypochlorite is starting from ordinary bleach, which already contains 4% or so of active chlorine. You add some NaOH
to the bleach and bubble chlorine through it. The chlorine can be made from calcium hypochlorite and HCl (or even better: TCCA and HCl).
|
The reaction equation for this is:
Cl2 + 2NaOH -> NaClO + NaCl + H2O
will the NaCl produced interfere with the haloform reaction in any way?
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
The NaCl should not interfere.
So what method did you use to make NaClO? Did you continue with electrolysis or did you make Cl2 gas and bubble it into NaOH?
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Long ago I had make an experiment with elecrolysis... It was about making some iodoform (it had a pretty nice odor and I wanted some...).
I checked it in thy Chemistry Lexikon of Hermann Römpp (pretty awesome book) and there was a description of the preparation of it I had made the
following recipe:
In 400cm3 water 60g potassium iodide 20g NaHCO3 80cm of alcohol or acetone (I used acetone) is dissolved an electrolysed at 65 Celsius. I used Pt
electrodes but not so expensive things are also good and it worked! Small crystals of iodoform settled to the bottom and more and more.... The only
problem was that because I used acetone, some iodoacetone was also produced what was not so funny...
But if the temperature is not so high hypoiodides should be formed or if potassium /sodium chloride is used, then hypochlorites.
It is strongly recommended to stir the reaction and to use small current to slow the electrolysis, but if the goal is to make some CHCl3, then the
original recipe will do it, just instead of KI use KCl/NaCl and instead of the acetone use alcohol, because chloroacetone is really bad for your
eyes
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|