genuinterest
Harmless

Posts: 6
Registered: 19-6-2011
Member Is Offline
Mood: No Mood
|
|
HBr in acetic acid from BCDMH
I have been considering how to make a HBr/Acetic Acid solution. Ideally one would add NaBr to acetic acid and conc sulphuric acid, unfortunately NaBr
is unavailable. After reading the various threads on producing elemental bromine, it seems BCDMH (1-Bromo-3-chloro-5, 5-dimethylhydantoin) may be a
viable replacement for NaBr, if HCl production can be avoided.
I was thinking that if a large amount of BCDMH was crushed and mixed with phosphoric acid, HBr would leave solution as gas and could be passed into
acetic acid.
The problem is that I think HCl would also be produced, maybe it would be recaptured by the excess BCDMH before it left solution, but maybe not.
Does anyone have any ideas to remove HCl from the produced gas if it is formed (without NaBr)?
Maybe pass gas through BCDMH?
[Edited on 13-6-2012 by genuinterest]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
UTFSE officially stands for Use The Forum Search Engine. Sometimes "Forum" is replaced by other
vernacular.
There's a quite adequate procedure already worked out, with experimental details, elsewhere on this site.
|
|
|
genuinterest
Harmless

Posts: 6
Registered: 19-6-2011
Member Is Offline
Mood: No Mood
|
|
I have read many threads about production of elemental bromine and a few posts on making Hbr. But I couldn't find the thread/post detailing how to
produce clean HBr from BCDMH.
Sorry, i guess.
Thank you for the suggestion Hexavalent but unfortunately NaBr is unavailable.
[Edited on 13-6-2012 by genuinterest]
[Edited on 13-6-2012 by genuinterest]
|
|
|
genuinterest
Harmless

Posts: 6
Registered: 19-6-2011
Member Is Offline
Mood: No Mood
|
|
I had a thought, HBr gas generated could be lead into a flask containing mainly dry NaOAc and a small amount of water or acetic acid.
NaBr is partially soluble in acetic acid, but maybe treatment with H2SO4 would remove most of it as NaHS04. I don't have my merck index handy...
|
|
|
genuinterest
Harmless

Posts: 6
Registered: 19-6-2011
Member Is Offline
Mood: No Mood
|
|
This is the idea i've come up with, will hopefully get a chance to see if it will work on weekend.
[If what I would like to be true actually was true] HCl from acid will displace the bromine from BCDMH making HBr, HCl from generator will produce HBr
in excess of what can be solved in H20 in reaction vessels 1 & 2. HBr and perhaps some HCl will react with NaOAc in vessel 3 producing acetic acid
and NaX, when the NaOAc is used up the HX will be dissolved in the acetic acid until saturation. Any HCl in solution will react with the formed NaBr.
When NaOAc in vessel 4 is moderately used up it will be reasonably certain that the acetic acid in vessel 3 is saturated with HBr. This can be
confirmed by titration.
Still mulling this idea over, and have yet to work out stoichiometry, but it seems like a reasonable method to me at this point.
Maybe people can discuss purchasing NaBr in a different thread?
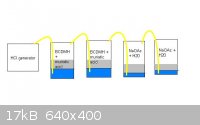
[Edited on 14-6-2012 by genuinterest]
[Edited on 14-6-2012 by genuinterest]
|
|
|
ScienceSquirrel
|
Thread Split
14-6-2012 at 05:22 |
ScienceSquirrel
|
Thread Split
14-6-2012 at 05:27 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
What is the reference for such an unreasonable claim? Since when is HCl a reductor strong enough to reduce bromoimides to bromides? I think you should
revise your knowledge of redox reactions if you have any genuine interest in chemistry. For the beginning it would do you good to actually read the
thread on the topic of bromine synthesis from BCDMH, rather to just pretend of having read it. Some members put a lot of effort into that discussion
and seeing how you manage to ignore it certainly doesn't please them. Then, after you read it and learn something, you might be able to devise a
reaction of BCDMH that could potentially give HBr as one of the products.
|
|
|
Nicodem
|
Thread Moved
14-6-2012 at 10:24 |
genuinterest
Harmless

Posts: 6
Registered: 19-6-2011
Member Is Offline
Mood: No Mood
|
|
you are mainly right. I have read those threads, but it is true my grasp of redox and chemistry in general is sporadic, i probably missed or skipped
over many pertinent details. Thank you for steering me back to text books, i will do as you suggest.
[edit] It appears i missed this thread: http://www.sciencemadness.org/talk/viewthread.php?tid=13380
when originally doing my searches. As nicodem said, there is much in there for me to learn from.
The other thread: https://www.sciencemadness.org/whisper/viewthread.php?tid=69... I will re-read and try to make sense of the things i don't understand.
[Edited on 14-6-2012 by genuinterest]
|
|
|