RIPPER
Banned
Posts: 17
Registered: 28-8-2004
Member Is Offline
Mood: No Mood
|
|
keto-nitrazines
keto nitrazines
I have a idea for energetic materials
with the O double-bound to the nitrazine-rings.
exambles:
triketo-RDX [(C=O)-(NNO2)]3->
1,3,5-trioxo-2,4,6-trinitro-2,4,6-triazine
tetraketo-HMX [(C=O)-(NNO2)]4->
1,3,5,7-tetraoxo-2,4,6,8-tetranitro-2,4,6,8-tetrazine
or
1-oxo-2,3-dinitro-2,3-diazine (C=O)-(NNO2)-(NNO2)->
and so on.

|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
There is nothing on "keto-nitrazine" on Google. There were 950 responses for nitrazine, which is apparently used in medical chemistry as an
alkaline pH-determining reagent, especially in testing amniotic fluid, and also urine and saliva.
John W.
|
|
|
RIPPER
Banned
Posts: 17
Registered: 28-8-2004
Member Is Offline
Mood: No Mood
|
|
ketos
keto = one
nitrazine = nitro aza
or nitro azine
examble:
RDX-trione
(Synth. of isocyanuric trichloride)
  
|
|
|
RIPPER
Banned
Posts: 17
Registered: 28-8-2004
Member Is Offline
Mood: No Mood
|
|
New Ideas
Some new ideas to energetic materials.
RDX-trioxide
2,4,6-trinitro-2,4,6-triazacyclohexane-1,3,5-trioxide
trioxinetrinitrate
2,4,6-trinitrotrioxine
trinitrocyclohexanetrioxide
2,4,6-triamino-2,4,6-trinitro-1,3,5-trioxine
triaminotrinitrotrioxine
triaminotrinitrocyclohexanetrioxide
  
|
|
|
Jome
Hazard to Others
  
Posts: 154
Registered: 10-6-2004
Location: Soutwest sweden
Member Is Offline
Mood: desiccated
|
|
By adding oxygens (how are you going to oxidise the aromatic rings?) you're adding weight without increasing the energy output.
|
|
|
jacKy
Banned
Posts: 3
Registered: 10-10-2004
Location: kÄckedei Hill
Member Is Offline
Mood: No Mood
|
|
Replys
I`ve found Sites at the E & W Forum
that writing gabbish to RIPPERS theoretical topic here !
nitrazine derivates
Whats your oppinion ?

|
|
|
David Marx
Harmless

Posts: 47
Registered: 13-8-2004
Member Is Offline
Mood: Intrigued
|
|
What does the last post even mean? "gabbish"?
The level of grammar has dropped dramatically on this site recently. Especially with all these schizophrenic posters!
alea iacta est
|
|
|
sylla
Alchimiste Belge Notoire
  
Posts: 110
Registered: 2-8-2003
Location: Belgium
Member Is Offline
Mood: No Mood
|
|
There is too much oxygen in triketo-rdx and this would probably give bad results (as an explosive) but monoketo-rdx seems to be very nice 
|
|
|
Reference
Anders Hoveland

Posts: 13
Registered: 12-7-2010
Member Is Offline
Mood: No Mood
|
|
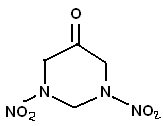
A little off, topic, but I was reading about dihydrosxyacetone, and its condensation with amines. "The pigment is the product of reactions between
dihydroxyacetone (DHA) and amino acids". I was wondering if maybe dihydroxyacetone would condense with the methylene dinitramine (MEDINA) ? Making
something like in the picture. Can't think of a use for it, but would probably be the most stable keto-RDX version.
|
|
|