| Pages:
1
2
3 |
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Only part in Kirk-Othmer that mentions nitration:
"Several electrophiles, such as acetic anhydride, nitric acid or alternative nitrating agents, such as ammonium
nitrate in trifluoroacetic anhydride (41), or sodium hypochlorite, react at N-1, which is followed by
reaction atN-3 under suitable conditions."
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Very interesting, that glycine H2N-CH2-COOH reacts so readily with urea to form hydantoic acid.
Structure (simple condensation product):
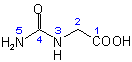
It should give interesting products with other amino acids, such as serine or threonine,

and introduce another OH group. Unless condensation occurs with the urea on the OH. Regardless, the products should give an interesting compound.
Nitration thereof should give a nice mixed nitramide/nitrate ester/nitrate.
Hydantoin,

which can be quite readily obtained by intramolecular cyclisation/amide bond formation (amidation) (see Axt's post above) is again interesting, not
only in the case of glycine as a starting compound, but for higher amino acids as well, all of which should give interesting nitration products. I
imagine using phenylalanine for condensation with urea, and subsequent cyclisation with HCl, to give the cyclic phenylalanine hyantoin derivative,
should give a very interesting and powerful nitration product.
I just wanted to point out the possibilities in this.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
| Pages:
1
2
3 |
|