| Pages:
1
2 |
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
For a good, friendly supplier, try http://www.hometrainingtools.com/ They've always had some of the less complex stuff at great prices, organic glassware, chemicals in small
amounts, etc.
|
|
|
thesmug
Hazard to Others
  
Posts: 370
Registered: 17-1-2014
Location: Chicago, Il (USA)
Member Is Offline
Mood: No Mood
|
|
As Dr.Bob said, many faucets are threaded. I faced this issue since I don't have any threaded faucets in my home. If you don't have any threaded
faucets, you might actually find that you can remove the end piece which is held on by, you guessed it, threads. Homesciencetools sells a condenser
faucet kit which is sized to accept both British (common even in the US) sink/hose threads and regular threads. It also comes with tubing to run the
water with!
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
Ok dr. bob. Thanks for warning me.
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
Dr.Bob in making ethyl acetate ( i just watched a video) they say set up a reflux. Do i have to do that?
|
|
|
thesmug
Hazard to Others
  
Posts: 370
Registered: 17-1-2014
Location: Chicago, Il (USA)
Member Is Offline
Mood: No Mood
|
|
Yes, you do. The reason is that in order for enough energy to be supplied to run the reaction efficiently you need to conduct it at temperatures above
the B.P. of the solvent. Of course you don't want to distill off your solvent, so you use a reflux setup to recondense the solvent.
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
How could i set up a reflux? I might get that distillation setup that Zts got So could i somehow set that up?
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
You can't really do a reflux with a graham condenser. You will flood the coils. A Liebig, reflux condenser, alihn, freidrich are all better suited for
refluxing. Read a few manuals in the forum library on basic laboratory techniques. The answers will come faster than bombarding everyone with one
liner questions that can easily be answered with google or reading books.
http://www.chem.ucalgary.ca/courses/351/laboratory/reflux.pd...
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
That link suggests cooling water should enter at the bottom but my personal experience is that entering at the top is better for reflux.
Ie. The bottom part of the column will be hotter than the top and it prevents vapor from escaping.
The Handbook of Petroleum Processing, Jones and Puhado 2006 has
a good diagram of how this is done industrially where the vapor is
condensed at the top of the tower and feed back via a reflux drum.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Well that would be the first time I have heard of somebody refluxing with the water running in the opposite direction than it should for a reflux (or
distillation).
By going from bottom to top you create a uniform jacket of cold water, when it runs in the top is just sloshes all over the place inside, but hey, if
that's how you feel it works best.
[Edited on 26-3-2014 by Mailinmypocket]
|
|
|
thesmug
Hazard to Others
  
Posts: 370
Registered: 17-1-2014
Location: Chicago, Il (USA)
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mailinmypocket  | Well that would be the first time I have heard of somebody refluxing with the water running in the opposite direction than it should for a reflux (or
distillation).
By going from bottom to top you create a uniform jacket of cold water, when it runs in the top is just sloshes all over the place inside, but hey, if
that's how you feel it works best.
[Edited on 26-3-2014 by Mailinmypocket] |
You're very funny (I'm not being sarcastic). If you do it on an angle will it work?
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Quote: Originally posted by Mailinmypocket  | Well that would be the first time I have heard of somebody refluxing with the water running in the opposite direction than it should for a reflux (or
distillation).
By going from bottom to top you create a uniform jacket of cold water, when it runs in the top is just sloshes all over the place inside, but hey, if
that's how you feel it works best.
[Edited on 26-3-2014 by Mailinmypocket] |
You have to restrict the outflow. It would not make sense to partially fill the column.
You also do not want the water to flow very fast.
|
|
|
TheAlchemistPirate
Hazard to Others
  
Posts: 151
Registered: 25-3-2014
Location: The point of no return
Member Is Offline
Mood: Enigmatic
|
|
Quote: Originally posted by zts16  | I bought a distillation apparatus last week. I got it in on Friday, and I've only used it once. It's not Pyrex, but seems perfectly acceptable,
especially for a beginner level one.
Also, it's a great deal and comes with all of the hardware required to support it, including an extremely heavy duty ring stand that I am very
impressed with. You can buy the exact same kit here:
http://www.cynmar.com/ProductDetail/11K21640_Distillation-Ap...
Also, the picture on there is kind of bad, so here's a better one that I took:
|
I wish I had seen this before spending 200$ and weeks of searching for a graham condenser distillation
setup 
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Is there anyone else who runs their condenser water from top to bottom? Well, either way that's not how it is done- working in professional
laboratories past and present, I have yet to see a colleague do it that way. In school the teachers would actually tell people they were doing it
wrong when they made that mistake.
Wiki
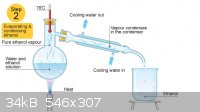
Quote: Originally posted by macckone  | Quote: Originally posted by Mailinmypocket  | Well that would be the first time I have heard of somebody refluxing with the water running in the opposite direction than it should for a reflux (or
distillation).
By going from bottom to top you create a uniform jacket of cold water, when it runs in the top is just sloshes all over the place inside, but hey, if
that's how you feel it works best.
[Edited on 26-3-2014 by Mailinmypocket] |
You have to restrict the outflow. It would not make sense to partially fill the column.
You also do not want the water to flow very fast. |
[Edited on 26-3-2014 by Mailinmypocket]
[Edited on 26-3-2014 by Mailinmypocket]
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Quote: Originally posted by Mailinmypocket  | Is there anyone else who runs their condenser water from top to bottom? Well, either way that's not how it is done- working in professional
laboratories past and present, I have yet to see a colleague do it that way. In school the teachers would actually tell people they were doing it
wrong when they made that mistake.
Wiki
Quote: Originally posted by macckone  | Quote: Originally posted by Mailinmypocket  | Well that would be the first time I have heard of somebody refluxing with the water running in the opposite direction than it should for a reflux (or
distillation).
By going from bottom to top you create a uniform jacket of cold water, when it runs in the top is just sloshes all over the place inside, but hey, if
that's how you feel it works best.
[Edited on 26-3-2014 by Mailinmypocket] |
You have to restrict the outflow. It would not make sense to partially fill the column.
You also do not want the water to flow very fast. |
[Edited on 26-3-2014 by Mailinmypocket]
[Edited on 26-3-2014 by Mailinmypocket] |
If you are refluxing like your picture it is definitely wrong.
That is distillation not reflux.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
No it's to demonstrate that water is supposed to always to in the bottom out the top. Regardless, I'm not going to continue disputing over a basic lab
skill that had been discussed a bunch of times on the forum and is demonstrated in textbooks. Do it how you like though.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2667
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
If you using a liebig or west condenser, you will always want to tun the water from lower to higher, whether rfluxing or not, as the water will not
fill the condenser otherwise. For MOST other condensers, that is also true, but there are a few coil type condensers that the water could run either
direction, especially the type with two coils in opposite directions, as the water will run both up and down no matter what. But they have a small
diameter coil, so the air in the line will get pushed out by the water.
In industrial systems, the water is rarely in a large space like a liebig, it is usually in a copper type line, which is wrapped around the reactor,
or in a very thin space around it, so the chance of building up air spaces is reduced, and typically the reactor is engineered/designed to avoid air
spaces in the water that way, so you can make the flow go what ever way you design it to work with.
And that is the key to using glassware, use it for what it was designed for. A Graham condenser is designed for condensing small volumes of vapor
into a small flow of liquid, typically in a vertical orientation. It is NOT designed for other uses, but many people want to use them since they
look cool. A Liebig is the simplest condenser and works well in many cases, and can be use for condensing, refluxing, or as a distillation column.
But some other condensers are best only for reflux, Allihn ones are ideal for vertical reflux, but pool liquid in them if used sideways. So use
Google or other sources and look at the item and see why it was designed the way it was, if you are not sure. The internet has made it easy to do
that now.
|
|
|
Ax165Xj
Harmless

Posts: 25
Registered: 31-5-2013
Member Is Offline
Mood: No Mood
|
|
I was getting ready to point that out. Coolant always goes into the bottom of the condenser!
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I bought the setup that copperastic linked in the first post of this thread, a few days ago, it should be here on the ninth. I will review it as soon
as it arrives.
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
Ok zyklonb, Thanks
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Ok, it came today - Several days after the expected delivery time. It was packed in such a way that it could have been dropped from the plane it was
shipped on without braking ! 5 stars for the packing. ! 5 stars for the packing.
It worked very well, although this is my first distillation apparatus so that statement is of course biased. All-in-all a very great deal - other than
the long wait from China. It says it borosilicate and it handled heat very well. It came with two thermometers - (It said it comes with one, so I
might have just gotten an extra.) They both go up to 200°C, which is great. It also came with about 6 ft. of rubber tubing for the water input and
output. The keck clamps were cheap plastic, and I broke on of them accidently, but if you careful, this shouldn't be an issue.
|
|
|
copperastic
Hazard to Others
  
Posts: 158
Registered: 15-3-2014
Location: In your basement
Member Is Offline
Mood: Good
|
|
ok ill probably buy one.
|
|
|
quantumchromodynamics
Hazard to Self
 
Posts: 67
Registered: 25-9-2013
Location: with much determination, nowhere in particluar
Member Is Offline
Mood: tired but still trying
|
|
check with Bob first
Gentlemen, I strongly recommend checking with Dr. Bob for any distillation apparatus before buying from China. Bob is an amazing guy, and if he can
help you, you will be very happy. The glass Dr. Bob has sent me is eclectic, much more interesting to learn from, some of it is the best Pyrex in the
business, seriously fun packages to receive. I bought two sets of glass and condensers from Bob and I can build like five different distillation
setups from the mix. I think experimenting with different setups is necessary for different reactions. Experiments are the key.
|
|
|
| Pages:
1
2 |