| Pages:
1
2
3 |
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Wow. I will definitely find some reading about this, very interesting. Thanks. I will visit a shop that I know and rummage, and will let you know
if I find anything, naturally.
[Edited on 12-4-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Phase transfer catalysis is very interesting indeed. Normally chemicals like tetrabutylammonium chloride are used, but it's not the only substance
that works, many other quaternary ammonium salts work, the dimethyldidecylammonium chloride is simply one I happened to notice in an OTC product and
so easy for amateur chemists to get their hands on.
The way it's suppose to work is that it forms dimethyldidecylammonium hydroxide in the presence of concentrated NaOH, but the quaternary ammonium salt
is quiet lipophillic, that is to say oil loving, and so dissolves in the oil phase pulling in the hydroxide ion as it goes where it can saponify the
triglyceride forming dimethyldidecylammonium oleate and glycerine. The phase transfer catalyst then shuttles the oleate ion back to the aqeous phase
where the sodium hydroxide solution sits and there exchanges this for another hydroxide ion and forms sodium oleate in turn and so the catalytic cycle
completes, on and on and on.
Long story short, adding a small amount of a fairly concentrated dimethyldidecylammonium chloride solution to your soapmaking mix should speed things
up... in theory 
You will have a small amount of contaminant by way of the phase transfer catalyst, but this should not be a problem for you intended application of
ferrofluids.
General Tip: MSDS's of products are often great sources to find out the rough concentration and composition of ingredients on commercial products that
may otherwise not report much on the bottle. Not always the case, but often so.
[Edited on 13-4-2014 by deltaH]
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
I just tried this with 1.533 g of NaOH (Elemental Scientific), 15.33 g purified water (my RODI unit), and 11.53 g extra virgin olive oil (local
grocery, Piggly Wiggly brand), mixture heated on stovetop at ~60°C with occasional very thorough mixing. It was at trace within 30 minutes even with
the overabundance of water which tends to goof things up. Are you heating yours at all?
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Some quick reading about this suggests that the soap making industry does not use PTC, and yet I did find references where it was being used. The
long and short of the impression that I got was that when used the reason given was mostly 'speed' and 'better yield' . Etaoin, Hi, The problem is
most likely the quality, and I did heat it up at the beginning, but never kept it warm. Even cold it should not have taken this long I imagine,
besides I would never have learned about PTC's. Maybe you should try your same product cold? If it takes only say 1 1/2 hours then we know I have an
oil suspect of being corrupted either purposefully or not.. Also ""and 11.53 g extra virgin olive oil"" I am sure that the amount you used has an
effect on time- I was using 300grams. The other day I did it with KOH and a different olive oil, that took only 15 minutes. (100grams).
[Edited on 13-4-2014 by CHRIS25]
[Edited on 13-4-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
If we assume the rule of thumb that for every 10C, the rate of this reaction doubles, then if Etaoin traced in 30 minutes at 60C,
CHRIS should hit trace at 20C in 8h 
This sluggishness also suggests that one has to be careful to make sure that the reaction runs to completion ultimately. I would definitely suggest
that you don't use a cold method of making soap for this one unless you plan to age your bars for weeks!
Slow cookers work well if you have one, alternatively you can try an oven warmer. You would want to aim to keep the soap at about 80C for several
hours after getting it to trace. Don't go too hot or the glycerine may decompose to foul smelling and toxic byproducts.
Alternatively, you can try a phase transfer catalyst if you can find a suitable compound OTC. But honestly, it just looks like you need some patience
and elevated temperatures.
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Ok Nicola, thanks. A learning curve in all this. But appreciations are on their way to the sunny tip of africa, although they might evaporate a bit
along the way. Fan oven seems good to consider, but will pop to the shop this
morning when it opens. After 12 hours covered the soap has thickened but I can still slide my finger into it quite easily. Thanks to Etaoin macckone
and HOH who have contributed. Fan oven seems good to consider, but will pop to the shop this
morning when it opens. After 12 hours covered the soap has thickened but I can still slide my finger into it quite easily. Thanks to Etaoin macckone
and HOH who have contributed.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
As I suspected and expected here in the land of sheep and goats, this major outlet has majority of its fungal and algal mixtures in spanish and
german, although some ingredients in English, none of them the Didecyl Dimethyl Ammonium Chloride, most of them have a butyl in their name, and
certainly no ammonium chloride. Anyway no matter. I can get 500mLs for 18 euros which is 50%. I presume this is perfect since it comes from a Lab
supplier. Is the 50% ok?
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Can't think why not, but only experimenting can confirm. Also if you're gonna go the non-OTC route, you may be well advised to search the literature
online for recommended phase transfer catalysts specifically for saponification (ideally also of long chain triglycerides). In this case, I'd be
shocked if it's not been extensively published and practiced already.
As for the thanks, no need, always a pleasure if I can be helpful.
[Edited on 13-4-2014 by deltaH]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
I suppose as a last note I started reading everything I could about this product and saponification using quartenary ammonium compounds. After 2 days
I am exhausted. I could not find a single document that mentions Didecyl dimethyl ammonium chloride in relation to any saponification process. The
only recurring thing was this product as a disinfectant to be diluted and put into many hundreds of cleaning products. The only references to
saponification I found were with DD ammonium bromide. But all of these were heavily analytical pdfs that were really way above my understanding.
Practically all the research led me to references to general quartenary ammonium compounds with analysis on all sorts of fatty acids. Too much for me
to understand. So Hot method next time and more patience. Or I might just bloody well buy the oleic acid, and I feel very defeated right now.
Thanks though, I have learned quite a lot.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Don't worry CHRIS25, when you're tackling problems like this, it can be tough when one is not following a known recipe (appologies if
this sounds patronising, it's not meant as such). It's normal to feel overwhelmed and dishearted, but when you have success, you will be equally
elated! May I suggest you take a step back and switch off from this for a while, I do this often and it helps to avoid a burn out with a particular
set of challenging experiments.
| Quote: | | The only references to saponification I found were with DD ammonium bromide. |
This sounds very good, the anion in this case shouldn't matter much. Can you attach that reference for us all to see please?
Don't feel defeated and make no mistake, what you are trying to do is admirable!
[Edited on 13-4-2014 by deltaH]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Yep I will certainly try again. In the meantime here were the two references, as vague as they seem to me. If they are completely off the beaten
track then please forgive me, I have no knowledge of organic chemistry and have little idea about what was being written. However, I am setting
myself the task to understand anionic and cationic surfactants and non polar and polar heads and tails - you get the idea, But I already have a
question and it is bugging me, having learned about this so far, I can not understand why citric acid is listed as a surfactant especially with ferro
fluid when quite clearly by definition it does not appear at all to qualify as a surfactant. If you do know, simple language to begin with thanks
ever so much, if you do not know (I know you are a chemical engineer though) then I will post this as a separate question.
http://www.ncbi.nlm.nih.gov/pubmed/11441600 (in the abstract)
http://www.google.st/patents/WO2010047705A1?cl=en (Second column claim number 4)
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I think I can answer your citric acid question...
While the term surfactant is commonly used to refer to soaps by most of us, they use it in a more specific way here because what they mean is that the
citric acid acts on the surface of the nano particles by adsorbing onto them and modifying its surface charge so that the particles repel each other
and form a stable colloidal solution. In this sense, it is a 'surface acting agent', but not in the sense you and I may commonly know or refer to the
term surfactant, yet this is nevertheless correct.
The theory behind this is somewhat complex and has to due with surface charge and zeta potentials.
A very big problem in preparing colloidal solutions, that is to say a suspension of very small particles that are stable and remains dispersed, is to
prevent the microscopic particles in the suspension from sticking together forming larger particles and ultimately settling out.
Surfactants play an essential role in stabilizing these particles and keeping them dispersed, in fact, in this context, they are often referred to as
dispersants.
Thanks for the articles:
The second one is of no use, it's about inorganic ammonium salts, phase transfer catalysts make use of organic ammonium salts, something called
quaternary ammonium salts in organic chemistry.
In that sense, the first article looks promising. While we are not very interested in the ultrasound (assume you don't own an ultrasonic bath), the
articles does investigate the use of a few phase transfer catalysts to speed up the kinetics of saponification. It would be useful to consider their
findings, I suggest you politely request this article in the publications section.
[Edited on 15-4-2014 by deltaH]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
I am doing quite a lot of investigation into fatty acids, it's proving to be an unexpected area of interesting reading for me. I have even mapped out
the stoichiometry for all these reactions, something I could not do a few days ago and one of my previous posts with this now seems embarrassing in
retrospect. Thankyou for responding to the citric acid.
The olive oil soap has hardened after three days, no residues or oils since I took a 19gram sample and it looked perfect, the ph is certainly above
11 still. Anyway I placed it in a 3M solution of HCl (Lab Grade SG 1.18), absolutely nothing happened, the soap just floated there quite happily even
after breaking it up. No dissolving, no precipitation, the acid had no effect. I am assuming then that I must wait some weeks for the PH to drop to
at least a 9 or 9.5. before the acid will break up the soap and the H+ ions from the Acid are able to take over where the Na+ ions were?
As for the first article I have no authority to ask this, especially since I do not know anything about what I am asking to be submitted, would people
in the organic chemistry dept be interested do you think?
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
| Quote: | | ...absolutely nothing happened, the soap just floated there quite happily even after breaking it up. No dissolving, no precipitation, the acid had no
effect. |
I think your castile soap is so hellishly insoluble that it's struggling to react/exchange ions with the HCl because it needs to go into solution
first. Don't forget, the salting out principle doesn't only apply to salt in solution, a fairly concentrated solution of HCl is just as ionic as a
salt solution because HCl dissociates fully in water by this reaction:
HCl(aq) + H2O(l) => H3O+(aq) + Cl-(aq)
So, what you might be seeing is the same thing as if you tried to dissolve a lump of your soap in 3M salt solution... it won't budge.
What I can suggest is to first try and dissolve as much of your soap as you can in clean boiling water, see how much goes in... hopefully it's usable
amounts. Then fish out what doesn't dissolve and while hot, pour that soap solution in into your HCl solution while stirring quickly. The ion exchange
reaction should be very fast and hopefully you will get your free fatty acid separating out. You may need to wait some time for the separation to take
effect and get two layers, you may just see milkiness at first because the droplets are too fine, hopefully they coalesce... (cue ominous music)
As for the publications, I was talking about the sub forum here in SM reserved for that purpose, you need to request permission from
Polverone though with a polite and short U2U message.
At any rate, if you got a hard bar after a few days, you're on your way, so no need to make it with a catalyst anymore.
Don't expect the pH to necessarily come down below 11, as I recall, you used a little excess lye. One can normally see this as it forms a powdery
layer around the bar after some time as sodium carbonate effloresces.
[Edited on 15-4-2014 by deltaH]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Finally!
Total amount soap made from 300g Olive oil = 419.2g
30.9g soap needed 200mLs de-ionized water to dissolve
Required 60mLs 6M HCl to precipitate Oleic acid (though 20mLs extra added to ensure everything was done)
Crude Oleic acid precipitated whilst wet 34g ( sticky and gooey )
Out of the chemicals that I have CaSO4, MgSO4 or Anhydrous NaSO4 would be suitable desicants.
Looking at images of Oleic acid on the web at least my colour is consistent. But could not find any anhydrous images.
Just been doing some calculations based on:
HCl + C18H33NaO2 = C18H34O2
Converting everything I did stoichiometry wise I find that I used:
0.1 mole Acid + 0.1 mole olive oil gave me 0.098 mole oleic
which confirms the above amounts dissolved but the yield is far far less than expected, (unless someone spots an error in my thinking)
Order of Images:
Well it was the only mold I could find
Dissolving
Filtered solution
Precipitation
Left over did not dissolve
Crude oleic acid - I hope
    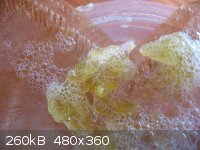 
[Edited on 16-4-2014 by CHRIS25]
[Edited on 16-4-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Nice photos CHRIS and well done! I was surprised to see that the product was a solid.
One question... what is the saturated fat content of the olive oil you used (you can find it on the label of bottle)?
If I understood you correctly, your yields are excellent, although they still probbably contain a lot of acified water in between the gooey mass.
It should melt when you heat it... does it?
| Quote: | Just been doing some calculations based on:
HCl + C18H33NaO2 = C18H34O2
Converting everything I did stoichiometry wise I find that I used:
0.1 mole Acid + 0.1 mole olive oil gave me 0.098 mole oleic
which confirms the above amounts dissolved but the yield is far far less than expected, (unless someone spots an error in my thinking)
|
I think you left out the NaCl on the RHS of your equation. Also, do you not mean 0.1 mole sodium oleate, instead of 0.1 mole olive oil?
Finally, by my back-of-the-envelope calculation, you only needed about 20ml 6M HCl (with a little excess) to exchange 32g sodium oleate (assuming your
soap is sodium oleate... close enough  ) )
[Edited on 16-4-2014 by deltaH]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Hallo, Firstly it melted within 25 seconds of entering luke warm water (in a beaker of course). The solid had the consistency of extemely thick hand
cream (if there is such a thing). Now it has melted it looks like melted ice cream. It can be poured albeit very slowly, though i boiled it for a
second or two and saw separation of water and what appeared to be a miniscule amount of oil?. I shall dry it with MgSO4 in a homemade desicator.
Yield? 1 mole HCl reacts with 1 mole sodium Oleate to give 1 mole oleic acid. Yep I missed my own pat on the back, wow.
The contents on the olive oil are:
saturated = 13.7g per 100mLs
monounsaturated = 71.1g (The oleic is here)
polyunsaturated = 6.4g
Right now I get it I will have to adjust all my calculations because this means that there was 213g theoretically in my 300g of oil used. Am
interested to see how it dries.
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Yes, you can certainly pat yourself on the back, yields appear to be excellent, though they are crude yields.
The reason I asked about saturated fatty acids is because they melt at a much higher temperature, 60-70C depending on chain length. Their presence
would raise the m.p. of your crude oleic acid and could explain in part, the firmness of the product, though you say the melting point is nevertheless
quite low... which is good.
WELL DONE AGAIN!
[Edited on 16-4-2014 by deltaH]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
well thanks nicola, and I did not know about having to melt it first. though I was aware of the meting point. what is m.p.? Yes the meting point
was literally luke warm water, I did not take a temp reading. though I will be doing next batch with extra precision. if possible in a kitchen, and
will purify the soap next time round with the salting out technique. Thanks so much for all your help, the learning process is far less stressful
when you get someone, such as yourself, giving little nudges in the right direction, then I can target the research and understanding more
efficiently.
""""Also, do you not mean 0.1 mole sodium oleate, instead of 0.1 mole olive oil""""? Yes I did sorry.
[Edited on 16-4-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Always a pleasure CHRIS and yes m.p is an abreviation for melting point.
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
oh
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
After 24 hours the off-white crude oleic acid had simply begun to turn into the same viscosity as a soft hand cream. It was supposed to crystalize at
room temperature after melting it, then placing it in a desicator with anhydrous MgSO4. Nothing, and the MgSO4 had not absorbed any visible moisture.
So this morning I placed it in my home made hot chamber with MgSO4, it started to melt again but the MgSO4 absorbed nothing. I switched to Sodium
Sulphate and within 10 minutes that became full of moisture, after just 30 minutes the crude Oleic acid melted into surprisingly a very clear liquid.
In the photo below, however, there are clearly two separations, the outer layer is completely clear and free of 'debris'? and gravitated towards the
centre you see an oily, globular clear mass.
Please could someone perhaps enlighten me about what is happening. My only thoughts are that the clear liquid on the outside is the pure oleic, and
the inner mass is a mixture of other fatty acids and the globular pieces are glycerol.
Important I forgot to add that the PH registered between 6 and 7 with universal litmus, so I used a comparitor strip and the Ph was below 6.8, meaning
that the Ph is definitely between a minimum 6 and maximum 6.8.

[Edited on 17-4-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
You should use a narrower container so that you can properly seperate your two layers by decantation. I would suggest adding a few drops of each layer
to clean water and observe what happens. I doubt though that there is any glycerol left in there. It could be that one of your layers is aqueous and
the other the free fatty acid or more likely perhaps, one is free fatty acids and the other triglycerides (oil). The simple experiment I suggested
should help in determining which.
[Edited on 17-4-2014 by deltaH]
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Thanks for responding so quickly. I went away for two hours and left the mass at room temperature. When I returned it had separated into a
whitish-light yellow crystaline and a clear liquid on top (not what it was when I first melted it, I believe now my melting was too quick and too high
perhaps). I poured away the liquid into de-ionized water and it dissolved meaning that the liquid was polar, the sight of the liquid going into
de-ionized water was the same effect and thing you see when you pour concentrated HCl into water, there is that appearance of something mixing then
complete clarity again, please forgive the amateur way of explaining, once I fully understand everything I see I can then experiment and refine the
procedures. I also tasted it, it was palatable and a bit sweet, I don't make a habit of tasting chemicals in the absence of analytical know-how,
but I reasoned that this had to be water after all with a little bit of something in it.
[Edited on 17-4-2014 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Yup, sweet and palatable sounds like glycerol. The optical effect of adding to water is also typical.
[Edited on 17-4-2014 by deltaH]
|
|
|
| Pages:
1
2
3 |