deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Nitrocreatine
I have been wondering for a while now whether creatine, the stuff body builders buy in way too big tubs  , might not be a
good substrate to nitrate and generate EMs. , might not be a
good substrate to nitrate and generate EMs.
Creatine has a nice combination of functional groups in terms of EM theory: a guanidine group (can be nitrated at least once comfortibly, see nitroguanidine) and an acetic acid group (can be destructively nitrated to a trinitromethyl group with loss of CO2, see tetranitromethane) and a methyl amine of sorts (good explosive 'fuel' group, for example see methylammonium nitrate in TOVEX).
A cursory search on 'creatine nitration', however, did not turn up much, but surely somebody somewhere has attempted to nitrate it?
The 'ideal' nitrocreatine compound I'm thinking of would be the tetranitro derivative:
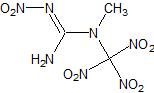
With a formula of C3H5N7O8
Note the excellent OB.
UPDATE:
Some preliminary computer modelled results have been volunteered to me (see attached files)
The .mol file is the result of a quantum mechanical minimisation without frequency calculation and it gives some idea about bond lengths and angles
and such.
Also a basic force field optimisation has been done on a P-1 unit cell to obtain a first guess as to the density of such a hypothetical structure (see
.cif file attached).
The determined density was 1.75 g/cc, not half unexpected nor bad for such an unsymmetrical EM.
I would guess that this would be a strongly brissant and sensitive EM, potentially making it a good secondary/booster candidate, e.g. tetryl
substitute (yes yes, already a legacy explosive and all that), however, I would also expect it to still be a rather toxic compound because of the
trinitromethyl group (analogous to the toxcitity of TNM because of the lability of one of its NO2 groups... taking note that the QM
minimisation did show a significantly longer bond length on one of those trinitro groups!)
Attachment: tetranitrocreatine (TNC).mol (2kB)
This file has been downloaded 803 times
Attachment: Hypothetical P-1 unit cell of TNC for density estimation.cif (3kB)
This file has been downloaded 840 times
[Edited on 24-4-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
with density in hand, you should also try to have the value of condensed heat of formation @ 298 kelvin so we can compute the detonation performance
of the explosive.
Dany.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Calculation of the solid enthalpy of formation would be very computationally expensive and is not an option (sadly) as it would require a quantum
mechanical minimisation of that unit cell file (currently it is only a force field minimised cell which is computationally easy and gives possibly
reasonable density estimations even if the bond lengths are a little off).
The next best thing would be to apply a group based emperical calculation of dHf as an educated guess, but I have never done something like this and
would appreciate some help/direction. Is there a specific reference you could recommend?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Have you got so far as theorizing a method for achieving this structure...
A quick look online shows pure Creatine monohydrate available @ < $20.00/1000g shipped in the USA.
Creatine on Amazon
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Yes the availability of creatine is very attractive/frightening.
As for a method... early days, but I was thinking to learn from the tetranitromethane methods developed specifically for acetic acid, as I believe
this is similar for at least that half of the molecule. For the other half one could learn from nitroguanidine preparation, but it would probably be a
case of whatever affects the destructive nitration on the acetic acid side would easily sort out the guanidine functional group?
The main worry is not have conditions aggressive enough to have the whole thing fall apart, for example, I am trusting the methyl group in that
molecule to hang tight 
[Edited on 24-4-2014 by deltaH]
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
I calculated the density with a molecular fragment addition method and got 1.77 g/cc, so rather close to the value presented in the first post.
I estimated its enthalpy of formation through a 3D structure drawing program called Avogadro. It optimises the structure and gives a rough estimate of
the ∆H. I chose the algorithm which gave the best results for trinitromethane, nitroguanidine and aminonitroguanidine and that gave me -25
kJ/mol.
The Kamlet-Jacobs equations give 341 kbar and 8810 m/s as detonation pressure and detonation velocity respectively. So this compound should be similar
to RDX in performance.
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
Can a Triazole ring be formed on the Guanidine end with treatment with HNO2 ?
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks Dornier for that excellent contribution. The predicted detonation pressure is remarkibly high  I will look into Avagadro for quick dH estimations then, thanks for the advice! I will look into Avagadro for quick dH estimations then, thanks for the advice!
Motherload, could you ellaborate please?
[Edited on 24-4-2014 by deltaH]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Sounds lovely- If it is possible to make and reasonably stable... You'll be right up there with Axt for subverting the nutrition supplement industry
into being EM raw materials suppliers!
I've been known to observe "Everything is actually for fireworks, if you look at things right!" while walking through hardware stores, drug stores,
farm supply stores...
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
My apologies ... Triazole is not the proper term.
What I was thinking of is a third =N-, (from HNO2, like in regular Diazotization) bond between the -NH2 and =NH resulting in a "Cyclo Azide" attached
to the Guanidine end Carbon..
My knowledge of chemistry is not advanced but I understand the basics.
Not entirely sure if a "Cyclo Azide" even exists ...
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Great, now how to make it? What makes it more interesting then ETN, stability?
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Motherload, if I'm understanding you correctly, the resulting structure would be a four member three nitrogen ring and so no, this is
unlikely, probably highly sensitive/unstable.
Ral, no idea about the stability as of yet, so I cannot comment about that definatively, though I am hoping that the guanidine group
would lend some stability although this is perhaps nullified by the trinitromethyl monster on the other side... who knows?
The how to make it is yet to be determined, as I mentionioned to Bert above, I know how I would approach a synthesis (based on
similar materials and their methods), but what would actually be a good recipe in the end would require experimentation.
As I've mentioned in another thread about collaboration, I am neither legally entiled to nor equiped to safely carry out such an
investigation, I hope that somebody, someday, who reads this AND satisfies those criteria may be interested to investigate this further and report the
results.
Please guys, leave this to experienced experts, exploratory EM research is very dangerous!
I would also suggest NEVER attempting anything in more than 10's of mg amounts to start out with until the system is well studied and it's
behaviour/tendencies understood.
Bert, you mentioned that you were considering gearing up for this kind of work, if you ever get there, I'll come and work for you

Also, you mentioned that creatine is sold as a monohydrate... that would need to be taken into account.
Perhaps a good start in this system is trying to prepare the mononitrocreatine first by [mild] nitroguanidine methods. That product should be
rather insensitive and not particularly energetic because of a terrible oxygen balance.
Step two could be a nitration of the mononitrocreatine in strong media (100% HNO3?) to form tetranitrocreatine (TNC).
Those are my hypothetical thoughts on it for the moment.
I believe braking down a multifunctional nitration into two steps would be most prudent in the beginning. One pot methods could be worked on once the
foundation has been laid and studied.
****
Wow, apparantly body-builders also use creatine nitrate 
I wonder if it's OTC in pure form, doubtful I guess.
Either way, easy enough to prepare the nitrate salt and then affect the dehydration for STEP ONE, namely, to prepare mononitrocreatine in the way one
would make nitroguanidine.
The question is can one nitrate it further to TNC afterwards?
[Edited on 25-4-2014 by deltaH]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
For sure an interesting case study.
It is not sure the trinitro moiety will occur the same way it does with TeNM from acetic anhydride nor the same way it does from the trimer of
cyanoacetic acid (cyclo-(-N=C(-CH2CO2H)-)3 what is a possible precursor of 1,3,5-tris-trinitromethyl-2,4,6-triazabenzene (or
2,4,6-Tris(trinitromethyl)-1,3,5-triazine)); this precisely because of the methyl group on the nitrogen.
This methyl amine group might be more or equaly basic as the formamidine part of the molecule...thus knowing if you will end up with a methylaminium
nitrate or with a aminium nitrate form the guanidine is a first step.
[Edited on 28-4-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
I remember something about creatine converting to creatinine in the acidic environment of the stomach. A nitrating environment would probably do the
same thing. Supplement makers starting selling the creatine ethyl ester to get around this problem.
en.wikipedia.org/wiki/Creatinine
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
PHILOU, I am not completely sure I understand what you meant with
| Quote: | | This methyl amine group might be more or equaly basic as the formamidine part of the molecule...thus knowing if you will end up with a methylaminium
nitrate or with a aminium nitrate form the guanidine is a first step. |
Can you maybe explain with some drawings please? Are you talking about the resonant structures of creatine nitrate?

If so, I think they are equivalent IMHO
Please elaborate, I'm lost 
****
Thanks gregxy, this is very interesting. I did not know about creatinine until now!
For those who have never heard about this, the structure from Wiki is:

This cyclisation might not be a bad thing. For example, the Wiki authors have forgotten one other tautomer possible with creatinine, IMHO:
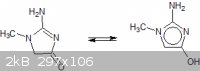
This kind of tautomerisation can be seen, for example with 2-pyridone.
This might actually activate the β carbon to further nitration because it is ortho to an ar-OH in a possible tautomer?!
[Edited on 29-4-2014 by deltaH]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by deltaH  | PHILOU, I am not completely sure I understand what you meant with
| Quote: | | This methyl amine group might be more or equaly basic as the formamidine part of the molecule...thus knowing if you will end up with a methylaminium
nitrate or with a aminium nitrate form the guanidine is a first step. |
Can you maybe explain with some drawings please? Are you talking about the resonant structures of creatine nitrate?
If so, I think they are equivalent IMHO
Please elaborate, I'm lost 
|
Not only about the resonant structure  . .
In a guanidine, you have 3 nitrogen atoms and each may be subject to an acid-base reaction... pKa1, pKa2 and pKa3 or pKb1, pKb2 and pKb3 are the
equilibrium constants of those reactions.
The acidity or the basicity of a nitrogen atom is dependant on its surrounding atoms and groups of atoms...often the electronegativity or
attraction-induction plays a big role; but also resonance structures.
CH3-NH2 is more basic than NH3 wich is more basic than NH2-NH2 itself more basic than NH2-OH
Cyclohexylamine is more basic than aniline.
So here you have:
1°)On one side H2N-C(=NH)- formamidine group in resonance what makes those two nitrogen equivalent linked to a nitrogen atom holding an inductive
methyl group and an electron withdrawing group CH2-CO2H.
2°)On the other side CH3-N(-CH2-CO2H)- linked to an electron withdrawing group formamidine.
The question is which one will display the highest basicity and be protonated in majority?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Okay, I think I am with you now, thanks for the ellaboration!
I have made this drawing for the one and the other attached below.

What was your thinking as to how this would affect the nitration?
[Edited on 29-4-2014 by deltaH]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by deltaH  | Okay, I think I am with you now, thanks for the ellaboration!
I have made this drawing for the one and the other attached below.
What was your thinking as to how this would affect the nitration?
[Edited on 29-4-2014 by deltaH] |
If the nitrogen bearing the methyl group holds the nitrate; then it cannot form a nitramine by dehydration without splitting the CH2-CO2H group or the
formamidine group away.
You would then end up with:
* H2N-C(=NH)-N(NO2)-CH3 in the first case (N-methyl-N-nitro-guanidine)
* CH3-N(NO2)-CH2-CO2H in the second case (N-methyl-N-nitro-glycine)
If one of the nitrogen on the formamidine side holds it; then you would get, after dehydration, part of your expected product
O2N-NH-C(=NH)-N(CH3)-CH2-CO2H <--> H2N-C(=N-NO2)-N(CH3)-CH2-CO2H (nitrocreatine)
If both possibilities are involved then you could have:
O2N-NH-C(=NH)-NH(+)(CH3)-CHCO2H. NO3(-) <--> H2N-C(=N-NO2)-NH(+)(CH3)-CHCO2H. NO3(-) (nitrocreatine nitrate)
or if splitting occurs
O2N-NH-C(=NH)-N(NO2)-CH3 <-->H2N-C(=N-NO2)-N(NO2)-CH3 (N-methyl-N,N'-dinitroguanidine)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Sounds like 'fun' 
...and on top of that, as gregxy pointed out, one might actually be affecting a creatinine nitration.
Thanks for your thoughts PHILOU, interesting as always. One can only hope cleavage will occur in the right place, but as always, some
bad cleavage is bound to occur even if unfavoured, selectivity will always be an issue with multifunctional exhaustive nitrations.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
It is possible but first you would have to convert the guanidine part into an amino-guanidine part.
This could be acheived by reaction of creatine with one equivalent of hydrazine... hydrazine will exchange for an amino group and evolve ammonia
gas...the later will be caught by the acidic part of the molecule.
H2N-C(=NH)-N(CH3)-CH2-CO2H + H2N-NH2 -heat/pressure-> H2N-NH-C(=NH)-N(CH3)-CH2-CO2H + NH3
--> H2N-NH-C(=NH)-N(CH3)-CH2-CO2NH4
[Edited on 3-5-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|