numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Extraction of Lycopene
This is a very easy extraction that can be done with all OTC products.
Lycopene is a type of Carotene (ψ,ψ-Carotene) found in tomatoes and has a very deep red color.
Methods:
Start with tomato paste, I used about 100ml. Add 200-300ml Acetone and stir very well. Filter, and throw away the liquid. Do this 2-3 times. This step
removes unnecessary contaminants and leaves a a pulpy red solid behind. Press the cleaned solid dry and transfer to a new container. Add around 100ml
of Dichloromethane and once again mix and filter. This time keep the liquid and set it out to evaporate.

You will be left with a very strong tomato smelling solid with a deep red color.
Notes: The Lycopene is not very soluble in DCM, so I had a major excess in Lycopene because to me, DCM is much more valuable than cheap tomato paste.
Different pastes will have different Lycopene contents which is why all the measurements are vague. Generally "an excess" doesn't hurt.
I used 'Royal select' brand tomato paste, and DCM was refined from paint stripper.
Will add a picture when dry.
|
|
|
Texium
Administrator
       
Posts: 4521
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Cool, I'll definitely try this, because I love the smell of tomatoes! I wish that there was a way to isolate whatever compound it is that makes tomato
plants smell the way they do though, because sometimes I just go sit in my garden and smell the leaves of my tomato plants for a while. Anyone have
any ideas regarding that?
[Edited on 6-14-2014 by zts16]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
This is a great extraction (I love extracting chemicals from household items, especially foods) and once I get my hands on DCM (which will probably be
never) I'll try this out! The only problem I have is that people are going to try this extraction and consume the lycopene because it's said to be an
antioxidant. So I'm going to put this out as a disclaimer:
DO NOT CONSUME LYCOPENE MADE THROUGH THIS METHOD.
[Edited on 15.6.2014 by Brain&Force]
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Texium
Administrator
       
Posts: 4521
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Haha, yeah, they probably would, even though it was previously soaked in DCM. Hopefully most people who use this forum would have the sense to not do
such a silly thing.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Quote: Originally posted by zts16  | | Cool, I'll definitely try this, because I love the smell of tomatoes! I wish that there was a way to isolate whatever compound it is that makes tomato
plants smell the way they do though, because sometimes I just go sit in my garden and smell the leaves of my tomato plants for a while. Anyone have
any ideas regarding that? |
Make sure you are coated in tick and mosquito repellant?
OK sorry but you must admit you set yourself up for that. It is the leaves, specifically the Trichomes which secrete a yellow goo responsible for the
smell you love. Just FYI but this chemical is what you need to work on extracting. I would create incense sticks so you can sit inside in the AC and
enjoy the odor without all the bloodsucking critters responsible for Lyme, West Nile, and God only knows what other nasty conditions.
From : http://www-plb.ucdavis.edu/labs/rost/tomato/leaves/leafanat....
"Trichomes occur in the epidermis of many plants. Trichomes are abundant in tomato plants. Here we see two types of trichomes found in tomato,
multicellular hairs and glandular trichomes. The glandular trichomes are responsible for secreting a yellow substance that gives off that
characteristic "tomato plant" smell".
 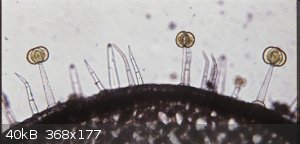
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Texium
Administrator
       
Posts: 4521
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Quote: Originally posted by IrC  | I would create incense sticks so you can sit inside in the AC and enjoy the odor without all the bloodsucking critters responsible for Lyme, West
Nile, and God only knows what other nasty conditions.
From : http://www-plb.ucdavis.edu/labs/rost/tomato/leaves/leafanat....
"Trichomes occur in the epidermis of many plants. Trichomes are abundant in tomato plants. Here we see two types of trichomes found in tomato,
multicellular hairs and glandular trichomes. The glandular trichomes are responsible for secreting a yellow substance that gives off that
characteristic "tomato plant" smell".
|
Yeah, that's actually what I was planning on doing. (Although there really aren't too many mosquitos where I live, luckily, it's too dry) And thanks
for all the info about it!
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Off Topic
I've GOT Lyme, courtesy of an Oklahoma tick. Shit has "settled" in my left knee and right elbow. It is no joke.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zts16  | Cool, I'll definitely try this, because I love the smell of tomatoes! I wish that there was a way to isolate whatever compound it is that makes tomato
plants smell the way they do though, because sometimes I just go sit in my garden and smell the leaves of my tomato plants for a while. Anyone have
any ideas regarding that?
[Edited on 6-14-2014 by zts16] |
First, I envy you for liking tomatoes, I hate tomatoes and actually wore a respirator, the only other times I wear it is when I work with Bromine.
Although, like IrC mentioned, the tomato plant smells different than a tomato, the lycopene smells more like concentrated tomato paste. Not sure about
the plant smell...
I should of said this, sorry for forgetting, but yes you are right, definitely do not consume this. If your that desperate to get the antioxidant
property, just eat tomato paste by the spoonful [ick]
Picture still coming... I didn't realize how bad the lycopene smelled at first, since the smell of DMC is omnipotent. Also the stuff is sticky when
dry, so I redissolved in DCM and transferred the liquid to an ampule and am letting dry in there so I can seal it without having to scrape the thin
film off the recrystallization dish. The stuff has such a powerful color, looking at the ampule it looks like Bromine without the vapor counterpart.
Here's a comparison:
Lycopene in DCM on the bottom left, Bromine on the top right.

And damn arkoma, I'm sorry to hear that; hope you get better. 
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Quote: Originally posted by numos  | | If your that desperate to get the antioxidant property, just eat tomato paste by the spoonful [ick] |
I LOVE DOING THAT
FOR REALZEZ
HOW CAN YOU FIND THAT DISGUSTING
The best idea would probably be V8 tomato juice, or just snacking on tomatoes.
Sorry to hear about that arkoma! I don't know too much about Lyme disease, but I hope you get better soon. Just hit it fast and early - the symptoms
become chronic after a while.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
unionised
International Hazard
    
Posts: 5105
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Lycopene is far too big and heavy to be volatile enough to smell. The odour must be due to impurities.
As for eating something which was extracted with DCM, you might want to look into the production of decaffeinated coffee and the artificially
caffeinated drinks.
|
|
|
Texium
Administrator
       
Posts: 4521
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
So I've been trying to find out what's in that yellow stuff that tomato leaves produce, and have found nothing. Does anybody happen to know what that
wonderful smelling chemical is that they produce? I'd really love to isolate it, and I have several tomato plants, I just have no idea of where to
begin. I have practically no organic chemistry experience.
Also, numos, if you hate tomatoes, what inspired you to do this extraction?
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | Lycopene is far too big and heavy to be volatile enough to smell. The odour must be due to impurities.
As for eating something which was extracted with DCM, you might want to look into the production of decaffeinated coffee and the artificially
caffeinated drinks.
|
I'm sure there are impurities, and I think I didn't wash with acetone enough which is the probable reason.
Edit:
Checking back, it seems like Lycopene is insoluble in water, perhaps a few washes with dH2O wouldn't hurt.
As for eating something that was extracted by DCM, yea DCM is fine if it's pure, it evaporates away and its gone. However my DCM was extracted from
paint stripper, and I am assuming most home chemists use that as a cheap source of DCM, and although it's fairly pure, I don't know what else came
over during the distillation. Also this is the same glassware I used to make the majority of my Barium salts.
Well I figured the only things I hate about tomatoes are the taste and smell... Since it will be locked in an ampule, these are not a problem. But
mostly I was on Wikipedia, and was reading a little about extractions, and I came across the carotenes which sparked my interest. (pretty colored
organic compounds!) Lycopene seemed the easiest to extract, and is also cheap. Now that I have a working procedure I will try again but this time with
β-Carotene from carrots (carrot flavored baby food maybe?). In essence it was cheap, easy, and I had everything to try it at that point in time.
[Edited on 6-15-2014 by numos]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
From Organic Experiments (alumina chromatography): Fresh tomato fruit contains about 96% water, and R. Willstatter and H. R. Escher isolated from this
source 20 mg of lycopene per kg of fruit. They then found a more convenient source in commercial tomato paste, from which seeds and skin have been
eliminated and the water content reduced by evaporation in vacuum to a content of 26% solids, and isolated 150 mg of lycopene per kg of
paste...These carotenoids are very sensitive to photochemical air oxidation. Protect solutions and solid from undue exposure to light.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Quote: Originally posted by zts16  | | So I've been trying to find out what's in that yellow stuff that tomato leaves produce, and have found nothing. Does anybody happen to know what that
wonderful smelling chemical is that they produce? I'd really love to isolate it, and I have several tomato plants, I just have no idea of where to
begin. I have practically no organic chemistry experience. |
Me either. I called it 'yellow goo' because I have yet to find the information. Obviously better to know chemically what it is in order to decide upon
an extraction method. Anyone know?
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Texium
Administrator
       
Posts: 4521
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
I went out to my garden to see if I could get a sample of yellow goo. Unfortunately, unless I was to use my entire tomato plants, I don't see how I
could get a usable sized sample. Once they are past their fruiting stage however, I'd happily tear them up and press them to try and get this yellow
goo. I tested it with one leaf today and got a decent amount of viscous yellow-green liquid, it had that characteristic smell to it.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I know that bromine water and tomato juice allows to make a chemical rainbow.
So pushing the bromination further might allow one to perbrominate the lycopène into all the 13 double linkages positions.
C40H56 + 13 Br2 --> C40H56Br26
The resulting perbromo compound maybe can exchange most (or all) of its bromine atoms for a nitrate group in strong AgNO3 solution.
C40H56Br26 + 26 AgNO3 --> C40H56(ONO2)26
The putative "nitro-lycopene" will be a solid explosive (of the nitroglycerin-nitroerythritol-nitromannitol family) with a density between 1,8 and 1,9
and VOD between 8500 and 9200 m/s, moderate sensitivity to shock.
C40H56(ONO2)26 --> 30 CO + 10 CO2 + 28 H2O + heat
Alternatively lycopene may be reacted with dilluted KMnO4 solution to add two hydroxy groups to all the double linkages
C40H56 -KMnO4 dill/H2O-> C40H56(OH)26
After extraction of the polyol, it can be nitrated with conc HNO3 or N2O5/DCM to provide "nitro-lycopene".
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|