quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
Selenic Acid from Se and H2O2, electrochemistry and other pure and efficient methods
the trouble here so far is with the first one, Se and H2O2
according to https://en.wikipedia.org/wiki/Selenic_acid ,
"One method of preparing selenic acid is by the oxidation of selenium dioxide with hydrogen peroxide: SeO2 + H2O2 → H2SeO4 "
the selenium dioxide is, according to https://en.wikipedia.org/wiki/Selenium_dioxide "Selenium dioxide is prepared...by reaction with hydrogen peroxide,... 2 H2O2 + Se → SeO2 + 2
H2O"
the attached paper mentions that "Other methods that have appeared depend either on the oxidation of selenious acid, a selenite, or selenate by means
of the electric current, or the oxidation of selenious acid with hydrogen peroxide"
it also mentions that "From the foregoing it appears that many methods for the preperation of selenic acid have been ; several of which seem to be
fairly simple. Actually, however, many of the essential details are lacking, even though upon them depends the success of the method."
OK, so it says that "When selenious acid or a selenite is refluxed for about three hours with 30 per cent, hydrogen peroxide, oxidation proceeds to
the extent of about 90 per cent. " on http://selenium.atomistry.com/selenic_acid.html <~this site is great, it has [hard to find]/[not found elsewhere] information.
original addition of a few ml of H2O2 (more data on that below) did nothing to 5.257g black Selenium powder, more was added and left in the same empty
seltzer bottle (it was washed out and rinsed with distilled water but the smell of blueberry remained unfortunately, note to self use plain seltzer
bottles if using seltzer bottles). ok, so that sat for a couple day/nights, it had microfizzed initially, the kind that you can hear up close, but
stopped over the first night it think, and i didn't notice any change.
then that was added to a quartz tube (domed on bottom), set on hotplate and then a set of 12v uv lamps was placed at its bottom (outside the tube on
the hotplate, on either side and tin foil tent/wrapped around (a square square was cup made by folding the fflaps to the side, crimped around the tube
and rubber banded))
these are the links to the 12v UV-C lamps
(http://www.ebay.com/itm/321437432790?_trksid=p2060778.m2749....) although (http://www.ebay.com/itm/UV-C-254nm-Germicidal-125mm-L-shape-...) seems to be a better option as it can be immersed in corrosive acids as the leads
are at one side.
it was surrounded by aluminum foil. also i hook the lamp up to 2 eveready 6v lantern batteries in series. usually this works but now i have 2 sets of
2 of them parallel because they are weak from other experiments and those 2 sets are in series, so 12v. the light seems to be bright as normal or
maybe a bit less, i don't remember.
this has been running and bubbling up in the flask. what i forgot to mention is that the H2O2 is 30% and seemingly decomposed somewhat. it didn't make
my skin white, while years ago 30% ecover did. the copyright on the bottle is 2012 so i think it is very decomposed, anyway, it was added in an amount
of between 350to500ml rinsing out the seltzer bottle and was decomposing slowly (it is still active) producing a foam on the top of the vessel with
the selenium. this continued with the UV light being run (i saw this when the stirrer was low/not on).
now this is being heated on the hot plate, and the heat is being raised towards 83-100 degrees celcius, (while platueing). in light of the data from
atomistry , i may get it to 115 to 120 degrees celcius, under the 130-140 degrees celcius which is said to decompose selenic acid. i am not using a
ground glass setup now and am using tin foil to help condense so if i reflux i guess i will have to move it to ground glass although i hoping to avoid
that as i need to pirahna clean the flasks i have and am seeing how well it will do with the UV and sub boiling for a while.
edit: it has been at least 2 hours and i have raised the heat from the 49°C to a bit under 70°C and have taken the thermometer back out to use the
stir bar, while i raise it some more, i have almost maxed out the range before full heat (the setting itself is noticeably higher it seems than the
last bit on the dial). (the hotplate itself is much higher than these temperatures, the flask is not heating efficiently)
edit: OK, so the selenium seems to be all gone besides a bit that is stuck on the wall. the liquid is transparent and seems to have a grayish hue to
it. it seems that it just became clear or it was when it was at 49°C, i dont know. the liquid has been wrapped with tin foil up and surrounding most
of the meniscus and has looked dark and the stirring funnel had made it look black at the top. so now without the stir bar going, it seems to be just
below simmering, or that is the hotplate making noise, haha i don't know, there is the tiny bubbles from fizzing still, a little more, and a larger
bubble every now and then (7-8or9mm or so). i am going to have to slosh it to get the last of the selenium into the water.
cool
edit: ok, did that. so now i have to see if it will turn colorless clear. i guess the UV might have helped, i like this.
edit: the smell is actually like creepy crawlers when you bake them. it smells stronger now. it has been cooling off for a bit and i noticed that that
is what it smells like.
let's make some selenic acid.
edit: wikipedia says selenic acid decomposes at 200°C. i dont know. the liquid still has a gray tint to it. this is puzzling, i don't know if this is
a colloid suspension of selenium that has reformed (is it reduced/ from reduction?)it is not much of the selenium if it is, and the solution is still
slightly bubbling (when i say that i mean a alot of fine bubbles at a steady rate all through the solution, it has been like this through most of the
reaction, more while heated, no burps now. at the beginning of the reaction there was not nearly as much bubbling i believe, not as concentrated and
at as fast of a rate)
it is actually looking more colorless and i notice that the rim of the meniscus on the opposite side of the tube looked yellow and noticed it was
refracting the color of some plastic on a table behind it. yep, actually it is seemingly totally clear and colorless.
i have yet to test the ph, although with the amount of H2O2 and H2O in there i suppose if there is any acid it will be not very acidic. hopefully all
of the oxide is now acid., so i think i will let it sit overnight, still covered, which i am happy about so no dust gets in.
edit: it turns out there is black powder on the tube bottom although seemingly not as much as was put in initially. i am going to let it sit overnight
and see if there is more in the morning.
Attachment: wi.wt1918.hhmorris.pdf (1.5MB)
This file has been downloaded 730 times
i delete the edited on list because i edit alot and it takes up alot of space saying that
[Edited on 20-11-2014 by quantumcorespacealchemyst]
[Edited on 20-11-2014 by quantumcorespacealchemyst]
[Edited on 20-11-2014 by quantumcorespacealchemyst]
[Edited on 11-21-2014 by Polverone]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
the black powder seems to be the same amount from last night so i am running the setup altered a bit to see if the powder will dissolve. the hotplate
melted part of the insulation of one side of a wire so i wrapped that with plastic wrap and set it up as in the pictures with the bulbs on the sides.
the lamps do not seem to be powerful enough to go through this tube but they at least do a little. if this setup used the single tube model (the
second of the two ebay links above) this can be immersed in the fluid. higher wattage lamps will probably have alot more effect. the lamps are said to
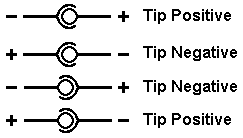
be designed to run on 12V 300mA. i am using a 9V 300mA wall adapter with a barrel plug which is + in the inside and - on the outside.the batteries are
low on power and the wall adapter lights the lamps well.
the pamphlet for the lamps/bulbs says not to reverse the polarity and to be aware when connecting. if using an adapter, this diagram is what to look
for on the device/power supply
from oli glaser on http://electronics.stackexchange.com/questions/33968/how-to-...
[Edited on 21-11-2014 by quantumcorespacealchemyst]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
i guess im behind on this. in 2006 i had access to 30% or 35% hydrogen peroxide bleach from ecover. i have assumed this whole time that non chlorine
bleach has been the same concentration. so when i purchased the about seven dollar bottle of it that i have been using in this reaction, from seventh
generation, i expected 30%H2O2. it turns out, that they sell 3-5% and ecover now sells 7% and it turns out to not be so readily available and
economic.
i have some left over food grade 12% i am going to add and see if it helps.
[Edited on 21-11-2014 by quantumcorespacealchemyst]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
from where did you get selenium
did you buy it on ebay or did you extract it from OTC materials
btw ,nice UV setup,using quartz glass tube and all
magpie,one of the members here also used UV in one of his synthesis in "prepublication"
but he used a mercury arc lamp
[Edited on 21-11-2014 by CuReUS]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Definitely interested in this. I was considering buying some selenium for an in-situ oxidation.
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
solution is clear and colorless
yes, ebay
i am hoping to get some more soon
the most economic source i found so far is from this seller, http://www.ebay.com/itm/100g-3-5-oz-99-999-Pure-Selenium-Se-...
there is a seller on ebay who sells these for $40, thick wall and charges a little extra to dome them, the seller said. happened to have one on hand
that was domed.
http://www.ebay.com/itm/Fused-Silica-Quartz-3-Diameter-x-12-...
thank you, i want to check that out, i didn't realize there is synthesis in that thread section
i had run the setup for a while at least 3 hours and then after adding the 12% (about 5 ounces to about 20+ ounces of the 3-5%) i let it set for maybe
3 more. the heat the whole time was higher than the first run that ended with powder. the hotplate was set just under the 140°C that came premarked
on the hotplate i have. (actual temp was not taken due to the setup). it was boiling lightly and surprisingly condensing enough on the walls and
aluminum foil cap, to keep it all contained. the interesting thing to me is that it may have actually been clear before i added the 12%. i am not
sure, as the tube had been dark from being foil wrapped and bubbling and when i unplugged everything, went to sleep and checked it on waking, it was
clear. i am hoping it is all selenic acid although it may be dissolved selenium dioxide and possible selenous acid from decomposion or a mechanism
that i don't know.
i am curious to know if UV with an arc lamp will work with only H2O by ozone production. as it is, it is unclear if the UV helped at all, as the
output was not immense. also i wonder if selenic acid is UV stable.
there are these 1000W mercury arc lamps for 89.77 USD from ARC
http://www.ebay.com/itm/ARC-DUV1000-30119-1000W-Deep-UV-Merc...
they run 30V 33.3A and will need a power supply. i am guessing a 1000W ballast (HPS) and voltage reducer/converter plugged to it (i am guessing power
loss through the converter will be minimal, i hope) will work
currently there are these 14.50 USD pieces which i am also exited about
http://www.ebay.com/itm/Power-UV-Source-250Watt-Ultraviolet-...
which run on 120V and are 250W, they have an interesting design where there is a third contact to start the arc which seems ideal. two of these can
seemingly be used with a 1000W HPS ballast if it is dimmable and does 50% power, 3 with 75% power.
there is also this 40 USD 250W 120V-240V ballast with free shipping
http://www.ebay.com/itm/HPS-MH-electronic-ballast-SG-250W-Wh...
i don't know about the HPS female plug layouts. tinkering will probably be required
and there are these unknown voltage 200W oshio lamps
http://www.ebay.com/itm/Ushio-USH-210EL-High-Pressure-Short-...
in the picture below there are two stir bars in there. it made less noise and i think stirred better than 1. the one stir bar seems to have a small
amount selenium powder stuck to it and looks a slight gray although most of this may be from the aluminum foil. the stuck on bits may have been from
letting it sit dormant which it did for most the time. i would stir it up every now and then for a bit. now i have to get it all evaporated, which i
will most likely not be doing tonight.
[Edited on 22-11-2014 by quantumcorespacealchemyst]
[Edited on 22-11-2014 by quantumcorespacealchemyst]
[Edited on 22-11-2014 by quantumcorespacealchemyst]
 
[Edited on 22-11-2014 by quantumcorespacealchemyst]
[Edited on 22-11-2014 by quantumcorespacealchemyst]
[Edited on 22-11-2014 by quantumcorespacealchemyst]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
selenic oxide precipitate
from leaving to stand with a cover for a while (since last post and before that when it was removed from the heat) there is a white/light pinkish
precipitate of what seems to be selenium dioxide which precipitated out slightly. it is probably due to the excess H2O2. i did notice small bubbles
around the stir bars which seemed to be growing and noticed bubbles rise to the surface every now and then.
this seemingly confirms that selenic acid was initially formed and the whole mass of selenium was converted. only after sitting was the dioxide
precipitated (which was on the bottom and suspended in solution when heated [the picture is while heating is being applied])
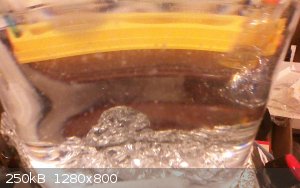
edit: it has been heating for a couple hours or so and i removed the uv lamps and took off some foil and saw what looked like particles but may have
been bubbles, as the foil was covering the outside of the tube up part the liquid line a bit. on heating longer and then turning on the stirrer, the
particles seem to have dissolved and may have been bubbles. the hotplate is now set to high to reduce the volume it somewhat. there is a small >mm
or so piece of sock hair or something in there i saw a bit after the start that i believe is a recent addition from carelessness.
[Edited on 11-12-2014 by quantumcorespacealchemyst]
[Edited on 11-12-2014 by quantumcorespacealchemyst]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
how to get rid of the peroxide safely and without contamination?
in two cases 3-5%H2O2 and with 30-35%H2O2 solution, if reacted to make selenic acid, i am only currently thinking of two ways that may work. one is
using a platinum catalyst, although this may react, so maybe iridium and then boiling down? if done with the 30-35% i guess it will have to be
diluted and then catalyzed and then boiled down.
the other is distilling of by vacuum which seems rather tricky, needing immaculate glassware. in this case i wonder if collecting it back in water is
an exothermic reaction or not for the reason of not having a storage apparartus/bottle for pure H2O2.
i am going for the reasonable option using some iridium.
if there are other ways, even if they are dangerous please let me know.
[Edited on 11-12-2014 by quantumcorespacealchemyst]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
If you want to get rid of peroxide, dilute it with plenty water (add it to water just to be safe, although I don't think the order will matter like it
does with acids) and put it down the drain. Peroxide will decompose to water and oxygen, so it's environmentally benign.
Don't add a catalyst to concentrated hydrogen peroxide; it's a bad idea (unless you want to make a rocket engine).
[Edited on 11-12-2014 by Cheddite Cheese]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
iridium added to catalyze H2O2 decomposition
ok, so i added some iridium, 0.079g and set up the lamps, covered in foil and ran the UV and hotplate set to a around the 120mark for a good while
and about halfway through this process i am describing, turned the dial up to around the 140mark (celcius). i just checked it and it has boiled all
the way down to about 10-20ml. there is a ring of off white precipitate on the bottom and on stir bar has what looks like some or all the iridium
powder caked on it. i don't know if the iridium reacted at all, and on attempting to remove a hair tha fell in, usin the thermometer to reach at it, i
deposited one drop of the liquid off the thermometer to the work surface and put some baking soda on it and it fizzed.
oh yeah, i forgot, i don't know if the H2O2 decomposed or concentrated alot so i turned off the hotplate before doing all the above and am unsure what
to do now.
i am getting the feeling the iridium dissolved, or that the amount was so small in the first place that that the remnant on the stir bar is all of it.
edit: this above post was originally an an erase and edit of the last post and it turned out to be uneditable.
i added the 0.079g Ir to the solution last night (after the starting solution was boiled till it was at 4/5 its volume) there was no reaction or
fizzing, i put the lamps back on and heated a bit then removed heat and left lamps on a bit, turned it off, slept and today i did the above procedure.
[Edited on 12-12-2014 by quantumcorespacealchemyst]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
Iridium selenate? selenic acid slowly dissolving iridium?
While this has been sitting as I haven't set up my vacuum pump or gotten take off adaptors yet, it seems to have darkened noticeably salmon.
it had turned light pink salmon, as can be seen here https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
and now it is much darker in color, stilkl alot of iridium (looks like all the iridium 0.079g)
It seems the acid has dissolved some iridium. This color has darkened enoughthat it seems a reaction progression is occuring.
the salts of Iridium being so colorful, is reportedly reason for the naming of Iridium after Iris and this seems to be a salt of it.
i thought the color may have been caused by this or another reaction due to something else listed after the pictures. i was leaning towards the other
mechanism, as i thought the iridium wouldn't react by only sitting. not now though.

The possible cause i thought possible besides the iridium dissolving was an organic volatile or acetyl chloride absorbing into the acid as the
aluminum foil seal is not air tight and the tube was sitting next to a reaction that was on hold, clamped next to it for a while.
The glass apparatus was sealed but disassembled and cleaned all but the flask, which was left to sit for a while longer and which i noticed gave off
smells of some sort. To me it smelled of sulphur or something related but the reactant used acetyl chloride which i believe was in exess was what i
expected. it's action on p-dibromobenzene (in presence of anhydrous AlCl3) was stated to produce a volatile brown liquid (after a workup). i noticed a
light brown volatile liquid had collected before the drying tube setup in the apparatus and soaked into the plugs used to stopper in the desiccant,
which were darker brown. i was curious to see if the brown liquid was the volatile product produced by the reaction (the light brown liquid being the
acethyl chloride which escaped the condenser before i had it running right and was condensed in the glass tubing where the color was brown days after
sitting). the stuff smelled of sulphur but had no sulphur, as far as i know, in the reaction. this puzzles me and interests me and wonder if the
molecules have just the right geometries/distances to set off the sulphur smell in nasal receptor sites. so i thought that the oil, and or acetyl
chloride where possibly attracted to the selenic acid and changed it's color and on standing away from the selenic acid tube for days it has darkened
in color and i don't think this is what happened (note, phosgene attracts to ammonia solution [making ammonium chloride and urea] so i guess it isn't
incommon).
this aforementioned procedure, part of the first part of "A Simple, Safe and Efficient Synthesis of Tyrian Purple (6,6′-Dibromoindigo)"
i bet it is fun to make. http://www.mdpi.com/1420-3049/15/8/5561/pdf
[Edited on 14-1-2015 by quantumcorespacealchemyst]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
it was red selenium precipitated out, does anyone know how make iridium salts?
The red solution it turns out was a red powder which settled to the bottom. I just found this out by rechecking it and swirled it to get back the same
red liquid. The iridium did not dissolve at all it seems. I do not know why the acid decomposed. May be from H2O2 still in it or a catalytic action
from the Iridium. Which doesn't make sense to me.
I am guessing the most efficient route to crystals of Selenic acid is reacting some exess of Selenium powder with 35%H2O2, filtering through glass
frit and vacuum distill/drying. Then storing in a PTFE capped bottle.
How are Iridium salts made?
[Edited on 3-2-2015 by quantumcorespacealchemyst]
|
|
|
Shivachemist
Harmless

Posts: 30
Registered: 1-2-2015
Member Is Offline
Mood: No Mood
|
|
I tell you a simple method here for preparing Selenic acid. First, we need to prepare selenium dioxide by burning Selenium in air or by the action of
nitric acid on Selenium. The aqueous solution of Selenium dioxide is Selenious acid which can be converted to Selenic acid by treating Selenious acid
with oxidizing agents like Bromine, Chlorine, Lead dioxide etc... or better preparing it from selenites. For example, selenites are formed when
Selenium dioxide is dissolved in alkaline solutions. Then you can obtain it by treating Silver selenite with bromine water (oxidizing agent). Silver
bromide formed in this reaction can be removed by filtration.
[Edited on 3-2-2015 by Shivachemist]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
Thank you, and ;nonetheless, I believe H2O2 is the purest way, besides possibly electrochemical means, with stoichiometric or a little more peroxide,
it seems the best way*, I have yet to set up for this. When I obtain H2O2 in ~35% concentration.
if platinum is inert to Selenic acid, perhaps dilution of solution, then catalyzing excess H2O2 (hopefully small) decomposition with Pt powder,
filtering, and boiling in vacuum/reduced pressure to evaporate water and then dehydrating the acid is possible.
I imagine a two neck flask, a drying tube connected to a vacuum take off adaptor on one neck, closed, and a specialized vacuum takeoff adaptor open
for distillation on the other. it has a PTFE stopcock with a hole the diameter of the flask and condenser (ie: 14mm flask, hole in stopcock and
condenser). pull vacuum, boil, close stopcock, open drying tube stopcock, let cool, close drying tube stopcock. I wonder how selenic acid can be
crystallized into single giant crystals and/or giant masses of geometric crystals. I hope it looks amazing.
[Edited on 9-2-2015 by quantumcorespacealchemyst]
|
|
|
|