Rhadon
Hazard to Others
  
Posts: 169
Registered: 26-5-2002
Location: Germany
Member Is Offline
Mood: No Mood
|
|
cheapest way to pure oxygen
One short question: What do you think would be the cheapest way to ~90%+ pure oxygen, preferrably free from reactive byproducts?
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
Obviously from decomposing chlorates or perchlorates is not a cheap option. From water (via electrolysis) is out of question aswell. I would say that
decomposing the H2O2 with MnO2 could be a cheap alternative, assuming that you can get technical cheap 30 % H2O2 (I can get about 3 litters of 30%
with 1 buck. That's roughly speaking around 175 grams (~122 litters) of pure O2/buck)
[Edited on 7-3-2003 by a_bab]
|
|
|
Rhadon
Hazard to Others
  
Posts: 169
Registered: 26-5-2002
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Yes, getting H2O2 is not a problem. I knew this method, but I thought that there could be a cheaper way, since H2O2 (30%) costs about 8 Euros / litre
for me. Anyway, thanks for helping!
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
Gentle heating of KNO3 would yield nitrite and O2, i think.
I doubt it would be possible to get one´s hands on industrial 30% H2O2 as a private person here...
Another disadvantage would be to get only very moist O2...
I can´t remember where i have seen it, nor if it was on this forum, or on E&W, but somebody described some experiences with liquifying air, using
a small compressor....fractional distillation of air seems far too complicated for backyard experimentation on first sight, but it is still the best
method for mass production....
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
I always dreamed about such a thing as making liquid air. The good news are that the nitrogen will boil first, leaving a very oxigen rich liquid. So
it'd be the cheapest method presuming that you are able to build such a device. It requires ALOT of tubing, that's for sure. IIRC, Linde
used a tower 10 m high and 200 atm presure. The most modern devices (Frankl) are using 6-7 atm pressure only.
|
|
|
Mongo Blongo
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
You can buy it for welding. I can't remember the price but it's quite cheap. It is practically pure and dry. It should be sold at almost any
hardware store. I will get some sometime (for purging NOx out of HNO3) and I will post the price if you want.
|
|
|
Rhadon
Hazard to Others
  
Posts: 169
Registered: 26-5-2002
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Although prices differ greatly between the US and the EU sometimes, it'd be nice if you could tell me what it costs. Thanks in advance!
|
|
|
Haggis
Hazard to Others
  
Posts: 238
Registered: 1-12-2002
Location: Mid-America.
Member Is Offline
Mood: Lacrymating
|
|
In a local hardware store, they sell Oxygen tanks next the the MAPP gas, propane and I believe butane. They are around a foot tall and cost 7.99.
There is no liquid in them so they feel empty. These tanks are very high pressure and you would probably need a regulator of some sort.
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
Once you have the equipment (in particular, a suitable power supply), electrolysis of water is cheap. Liberating 1kg of oxygen from water requires
3.7 kilowatt hours of energy (assuming 100% effciency). Even allowing for a lousy efficiency, this is cheaper than decomposing H2O2.
Chris
|
|
|
Rhadon
Hazard to Others
  
Posts: 169
Registered: 26-5-2002
Location: Germany
Member Is Offline
Mood: No Mood
|
|
But then you have a mixture of oxygen and hydrogen, which you won't be able to separate the easy way.
|
|
|
Aaron-V2.0
Harmless

Posts: 18
Registered: 19-9-2002
Location: Washington.
Member Is Offline
Mood: No Mood
|
|
IIRC in electrolysis the H2 is released at the negative terminal and the O2 is released at the positive terminal? Possibly the other way around. So
knowing that simply use a cell that's U shaped with the anode/cathode on each side so the gas is not mixed.
I think.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
make your electrode hooked and put two tube over them. (Maybe my electrode are inverted)
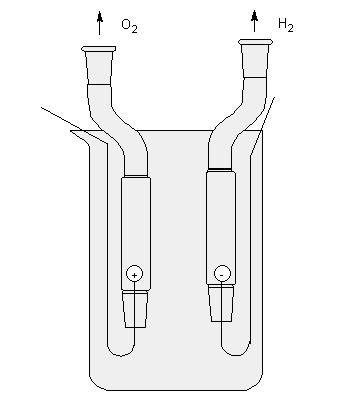
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
Mongo Blongo
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
They cost 6.99 from a chain store called Halfords. It sounds like they are exactly like the same ones that Haggis described and they do need a
regulator. The regulator I got was 11.99. They got oxygen, CO2, propane, acetylene, argon and other useful gases that I can't remember, all pure
and dry to boot.
|
|
|
Rhadon
Hazard to Others
  
Posts: 169
Registered: 26-5-2002
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Sounds nice, maybe I might take a look at it. Thanks for informing me Mongo, also for the electrolysis hint from Blind Angel and Aaron-V2.0  ! !
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
What an old thread! Recently I ran across a mechanical device that separates the nitrogen and oxygen in the air. I think the concept is simple
enough that it could be done at home.
http://www.casi-ne.com/nitrogen.html
Hodges
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
Check the obituaries for some old geezer who was on a 'concentrator' ;-) The things can easily produce 4+ liters per minute of O2. My mother is on
one and the thing puts out lots of O2 from room air and electricity. It works by selective adsorption of O2 or removal of the N2, I don't know which.
I've seen commercial ones at second hand machine part warehouses that were used to supply welders and cutters.
|
|
|
Twospoons
International Hazard
    
Posts: 1321
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
The hard part is getting the adsorber, the rest is just a compressor and a couple of valves. I did see a patent once that used an adsorber based on
burnt coconut shells, modified with ethene(?) to reduce the pore size. Just another form of activated carbon I suppose.
As a bonus to your O2 supply you also get a nice stream of fairly pure Nitrogen.
There's another system that uses reverse osmosis, but the cartridges are really expensive ($2000 for a small one!).
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
I'm seeing oxygen concentrators as low as $70 on E-Bay (Buy-it-now for a 3 liter per minute used unit).
I don't have much need for generating large amounts of oxygen at the moment but I needed to generate a lot I think this would be the way to do it.
They all seem to consume 300 to 400 watts so that is going to cost only a nickel or so an hour to operate. That's about 1 penny per 40 liters of
oxygen!
Hodges
|
|
|