| Pages:
1
..
15
16
17
18
19
..
48 |
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Rosco Bodine
After you have baked your spinel interface and MMO coating , maybe dip the anode into a Bi / Sn Pytlewski polymer and then simply dry it, or only dry
the final coat
if earlier coats of the polymer have been baked. Then put the anode into the plating bath and apply the PbO2. |
This seems certainly worth an experiment. The trick is going to be to scavenge oxygen out of the Ti passivization layer and clear it
out. An ionic conductor seems ideal for that, since it gives the oxygen somewhere to go instead of being trapped under a layer of coating. This leads
to the following suggestion: electrostatic firing. Put a negative electrostatic potential on the Ti. This creates an electric field in a direction
away from the surface so that O<sup>2-</sup> ions can migrate away from the surface.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
If you are talking again about cerium, I really think that angle is something which would have to be explored on only the outermost working layer,
nowhere near the substrate.
There really need be no worry about the oxygen bound to the oxide at the interface, where that compound is a spinel
and is basically desired to be impervious to ionic oxygen.
The dopant is what provides conductivity there, not by any
ion flow or exchange, but pure electron flow, just like those
little desk toys with the array of metal balls which are allowed to swing, and one impacting the array from one side
causes another to leap away from the opposite end of the array.....that is how the quantum jump and electron flow
would likely occur, the metal balls of course being analogous to the outer orbital electron of the doped conducting layer molecules.
The adhesion principle is something I thought could likely be useful even between subsequently applied layers of MMO, using the Pytlewski polymer for
a dip and dry wetting agent.
A simplistic example of the adhesion principle is illustrated
by what happens if you lay one polished glass microscope
slide on top of another. With only air between them, it is
easy to lift one from the other. Now try putting a drop of water between them. Now suppose instead of water, sodium silicate or sodium hydroxide, and
heat the sandwich. There can be a kind of diffusion bonding effect
with the solvent effect of oxides at pyrolytic temperatures,
where there is a sort of transition from one bonding scheme to another, like solvent welding plastic laminates.
This is a parallel sort of adhesion scenario which could
be of usefulness in anode coating schemes. But it hasn't really been reported or described in the literature I know about, so I'm not certain it will
work...it just seems like
a good chance that it would.
[Edited on 23-12-2008 by Rosco Bodine]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Rosco Bodine
If you are talking again about cerium |
No, I wasn't. Bi-doped tin dioxide is also an ionic conductor, as I
recall.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It's a conductor, but an ionic barrier to oxygen, I'm pretty sure. That is what made me think you were advocating cerium there. There are other
things which would work
probably besides Bismuth, whatever fills the SnO2 lattice
in a way that leaves no room for ionic oxygen to slip through the open areas. It is good if it will do the same thing with TiO2 also. Basically what
you are wanting is to create an electron conductive "sub-molecular (ionic) sieve" which has a mesh opening too small for an oxygen ion to get through,
so oxygen ions hit a wall there at
the enginereed barrier.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
While waiting for a break in the WX (it is COLD) so I can actually plate, I began a reference document mainly for my own use. It combines the
knowledge of several patents into a methodology that will hopefully work. I'd be pleased if guys here might comment on it, especially if they find
something in error. It is about 80% done:
http://www.5bears.com/perc/pbo2_01.doc
The remainder of it will have a "recipe" for a hopefully successful plating.
[Edited on 23-12-2008 by Swede]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Aren't you planning to use some sort of interface coating on the substrate before you plate on the PbO2 ?
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Rosco Bodine
Aren't you planning to use some sort of interface coating on the substrate before you plate on the PbO2 ? |
Yes, initially it will be some of the MMO mesh I have on hand. But there's been nothing that I've read that should prevent plating over any number of
substrates, unless all these patents are bogus.
The nice Alumina guy is going to send me some samples (3 grades) of AlO(OH), each with varying characteristics... crystal size, pore volume, etc.
Should be interesting!
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
No comments on my .DOC? It's simply a meshing of multiple patents re: PbO2 deposition, averaging values where needed, and trying to scale what is
really a too-large industrial process, down to a home lab size. Larger plating systems have better chemical stability than smaller ones, and will
probably do a better job, but just what size that might be is unknown.
Hopefully as plating starts and results come in, I'll be able to add to the document with some definitive observations.
The initial test setup will use some pretty small test anodes, and with the cathode at 2X to 3X the current density of the anode, I needed to make a
pretty small Cu cathode that will also contain a 100 watt cartridge heater. The best form to present to a rotating anode is a hemispherical one, so I
took the cut sheet, bent it, and soldered it (lead electrical solder) to the Cu tube that contains the cartridge heater.


For cathode "area" I am counting only the area of the Cu sheet facing the anode. In this case, it is about 30 square cm. The test anodes have an
area of about 60 squared cm.
At the top is the connection for the system, and the leads for the 100 watt cartridge heater. This rig should be able to heat up a liter to 70
degrees with ease.

If the weather cooperates, I am going to mix up about a liter of plating solution and at a minimum set things up before I have to go back to work.
One thing I read that was interesting was a "thermal shock" effect... you want the anode to be at the correct temp prior to turning on the power, yet
you don't want it immersed in the plating solution to heat up, so probably the best answer is a beaker of DI water at 80 degrees or so to pre-warm the
anode after initial prop, but prior to plating.
[Edited on 25-12-2008 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
You beat me to the mark with your last post. So some of my ramblings are similar to what you are going to do anyways.
What is below is some suggestions/thoughs.
From a previous post by Alan:
_________________________________________________________
So here is one surfactant I'm looking for,
Cetyltrimethylammonium bromide, CTAB
hexadecyltrimethylammonium bromide, HTAB
CAS No: 57-09-0
Molecular formula: C19H42BrN
Synonyms: acetoquat ctab, cetyltrimethylammonium bromide, CTAB, bromat, centimide, cetarol, cetrimonium bromide, cirrasol-od, cycloton V, pollacid,
quamonium, softex kw
___________________________________________________________
CTAB has been reported to reduce stress in electrodeposited LD as well as getting rid of bubbles etc.
Weigh the substrate before coating. The density of LD is around 9.37 grams per cc. It may be useful data later.
Consider not using surfactants and use mechanical stirring/vibrating etc or use beads.
Keep an eye on plating efficiency and perhaps add Hydrogen Peroxide. (US 2994649). The build up of Nitrites may give bad coats. Dont know myself. The
patent used a 300 gallon tank (!!!!!!!!!!!!!) but did not give details of amount of LD plated from tank between additions of Lead Ion
replenishment/neutralization compounds etc.
Don't put any more coats other that LD (let it be Alpha or Beta) on top of the MMO coating. KISS. You have purchased Titanium with an interface coat.
Use that and see how it goes. When you have established that perfectly good anodes are possible/not possible with what you have,
then you can go on to do piles more coats of other stuff if you wish. The anode will work OK IMO with Ti + MMO as substrate (purchased) with LD on
top.
Regarding thickness of coating. PbO2 does wear. A thick coat will give a longer lasting anode. Commerical Graphite substrate anodes had 5mm thick
coats and lasted a year or two (as far as I can remember reading). With the Ti substrate you can wear nearly all of the LD off before declaring the
anode worn out. With Graphite substrate I would presume they would have to replace
anode(s) when coating got thin* or if Graphite started to appear in the solution from a failed anode(s). (*how thin I have no idea).
0.02 inches (0.5mm) as a coating on your MMO is far far too thin IMO. Make the anode (at least) approx. 7 mm thick, thats approx. a coating thickness
of 3 mm. If you fill in all the gap areas in the mesh it is going to be that thickness anyways.
Cracking/flaking PbO2 is not going to be a problem with a mesh sustrate.
LD will wear less in pH controlled cell than non-controlled cells...............we think/hope.
The patent that made seperated LD 'islands' on the Ti substrate where rather large anodes and probably far bigger than you are going to make.
Addition of Cu Nitrate. It has been pointed out elsewhere that if you use Copper Cathodes then you will not get Lead metal being deposited on the
Cathodes (wasting Lead ions). You could try starting off without Cu Nitrate and if Lead starts to appear on Cathodes then add some Cu Nitrate
solution. I have always wondered about the Cu in LD. Does Cu get trapped in the LD?? I have no idea.
Cu is bad news in Pyro struff sometimes.
The Nickle and Bismuth I dont really know. The Bismuth seems to be an additive for primarly improving wear resistance at low temperatures only (as
you said) mostly in Hypochlorite prodution. The Grain refining and efficiency? are a bit vague (IMO). Fig 2 is for Hypochlorite production.
The commerical operations, reported both in patents and scientific literature, that used LD for making large scale amount of Perchlorate (and
Chlorate) did not use Bi (assuming they are not telling porkies). They got good current efficiencys. There is not a lot of room for improvement in CE
(making Chlorate/Perchlorate) when going from plain LD to Bi/LD, IMO.
I am inclined to want to try these thing with as few additives, coats etc so as to keep it as simple as possible. Test for a few months and see how it
goes. If the anode fails, (I do not think it will fail with what you propose to do anyways) you will be wondering would a plain old 'vanilla' Lead
Nitrate bath have been wiser.
Also, for the sake of the armies of poor Garage Chlorate and Perchlorate making Guru's following in your footsteps that may not want to shell out
their precious cash (credit crunch now, with the 'END' just around the corner remember!!!!) on Bi, Ni etc.
The large tank in relation to anode size that you intend to make is your greatest strength. Most Garage makers would not have a tank that large.
Try not to make too big an anode as you will negate the advantage of the big volume that you have.
I am inclined to put emphasis on seeing if an idea will work using a minimum(ish) (at least not a lavish outlay) of stuff as
opposed to being hell bent on obtaining a large anode that will work for the rest of my days. Making a small anode
(say 1.5cm by 10cm by thickness) will test a scheme as will as one ten times that size. I am not suggesting that you are
hell bent on a hugh anode but it is a temptation that is hard to resist, especially with that hugh square of MMO'ed Ti.
If a particular idea and size of anode can be made consistently in a liter bath then you can always get larger and better later (easy to say when you
are not actually doing the stuff). That's just my bent. Back of the ditch stuff, field expedient or whatever you like to call it. Since Ti is
becoming more available (bikes, camping stuff etc) it is no longer an impossible-to-get exotic material. Ti + MMO not so easy though. Perhaps the
bike grades etc (Ti alloys) are not suitable.
The patents (when one tank is being used or even two) always seem to be very tight liped on the size of tank(s) that was used and when they do
mention tank size they are vague as to the anode size and amount of LD they plated from the system at a sitting.
They are never tight liped on the brillance of their latest creation (anodes or otherwise). Best ever, better than anything before, vastly superior,
faster, cheaper, sweeter, longer, more efficiency, mother of all anodes/or whatever, more graceful, more devine, more ambiable, more mercyful, more
remarkable, couldn't be beaten with a big stick, more advanced, at a 'higher level', incorporating all previous discoveries/visions only understood
by those intellectuals and practitioners skilled in the particular advanced art of anode (or whatever) making, more glorious, more gallant,
panoramically visioned method, less overvoltage (nearly forgot that one!), greater CE, uses less materials, etc etc etc etc. [feel free to add, this
is only a tiny list]
When we go to use some of the patents in a partiular situation (say limited funds) then some of them are hopeless or worse.
The Ti substrate should give the Garage Chlorate/Perchlorate maker a definite achievable LD anode unlike the Graphite or massive which has not worked
by and large.
With the set up you have (10 Liters), I would be surprised of you could not obtain servicable Graphite substrate anodes but my 2 cents worth on the
GSLD Anode would be not to touch it with a forty foot barge pole!!
About the thermal shock. I once heard of a guy who put a home made LD Anode from a hot Chlorate or Perchlorate
solution into a cold one (or the other way around) and it cracked there and then.
Something a bit sneaky.
In order to throw some light on the 'will MMO make Perchlorate' thing, if someone was to contact those
folks who make MMO, Platinum, LD anodes and ask for a quote on an MMO anode for Perchlorate. Insist that you do not want a Platinum or LD anode. If
they can deliver then I guess MMO (the proper stuff) is a useful Perk. anode.
I have a feeling that they will be trying to flog you a Pt anode though.
Attached is yet another Thesis. Definitely bed time stuff perhaps after you have fell asleep!! It is rather indepth stuff.
On page 53 it says that switching MMO on and off in a cell wears it alot.
edit:
Could not attach File as it is too large. Link here.
Aspects of Electricochemical production of Hypochloride and Chlorate (1989)
http://alexandria.tue.nl/extra3/proefschrift/PRF6B/8908379.p...
There are four more here. (including the artical above)
http://en.scientificcommons.org/lr_czarnetzki
And just to indulge you all even further. There is some good reading below about pool chlorination/disinfecting anode below.
Definitely worth some time to take a gauke IMHO at this one.
Pt and Diamond and Graphite anode are hopeless Chlorine producters at very low Chloride levels. You need MMO.
They tested some MMO in Berlin tap-water too. Now there's a speciality chemical!!!!!!!!!!!!!
http://www.platinummetalsreview.com/dynamic/article/view/52-...
Over and out,
Dann2
[Edited on 25-12-2008 by dann2]
[Edited on 26-12-2008 by dann2]
|
|
|
granitestaterecovery
Harmless

Posts: 26
Registered: 25-12-2008
Location: beside myself
Member Is Offline
Mood: combative
|
|
In response to JPSMITH123 try placing graphite anode in lambskin condom Then if you would respond to me personally. you are pretty consumed in your
interest.
Graphite
This is probably the cheapest and easiest anode material to make *chlorates* with. According to what I've read in some
patents, getting a yield of 100 kg of chlorate will, depending on conditions, erode between 0.4 and 1.0 kg of material from an *untreated* graphite
anode. If the graphite is impregnated with various resins to make it "waterproof", the loss can supposedly be reduced to 0.1 to 0.2 kg per 100 kg of
chlorate.
Supposedly graphite will make perchlorate, although I've found hardly any documentation to that effect. I assume it will do so at low efficiency under
relatively extreme conditions, e.g., low chloride and high chlorate concentration at higher voltage, compared to other materials.
In fact, if it is desired to make chlorate that is free from perchlorate contamination (especially in a "continuous"
process), graphite may be the material of choice, since all of the other materials seem to be much more likely to make
perchlorate under a given set of conditions.
I should say I have some ideas on how to treat graphite to make it better, and I'll probably post these ideas in the future.
Granite State Recovery & Recycling
All our scars in life are self inflicted
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Dann2, as usual, you're a good voice of logic and reason.
I tried to edit the previous link to the .DOC but apparently the ability to EDIT vanishes after a certain point. The current document (and I'll try
to keep this link active while I work on the doc:
http://www.5bears.com/perc/pbo2.doc
Very, very few of these patents use a single-pot plating process. There is almost always circulation, with a fresh supply of electrolyte flowing into
the plating chamber, and "used" electrolyte flowing out, to be reprocessed, recharged, refreshed, whatever magic they do to achieve their "rock-hard,
ceramic-like" PbO2 coatings that never, ever seem to fail. Those guys could plate a dog-turd, and it would produce perc for years, with only a
0.00001% loss of material every 1,000 amp-hours.
While a complex, circulating system is possible in an advanced lab, there are maybe 3 guys who would bother, just for the sheer joy of the complexity.
I'm one of them, but I'm not going to do it. The idea is to come up with a one-pot process that works reasonably well.
I've seen remarkable consistencies among maybe 6 patents with regards to reagent concentrations and current densities. These consistencies are what
I'm trying to ferret out, because it implies a successful value or methodology. Some of the stuff is way out there, and a lot of that I am ignoring.
I tend to agree on the Bismuth... the nickel, I'm halfway convinced that the grain refinement it MAY add might make it worth it, but the beauty of
this bath is that we can always add stuff... removing it may be hard to do, so best to test with simple solutions.
I'm at a sort of fork in the road... I've got a large setup, but I can easily execute a small setup to "save" chemicals. By going immediately to a 6
liter bath, and working modest anodes of 8cm x 4cm, the chemical stability of the bath will be improved. But if the bath gets polluted or ruined,
it's a big waste.
I think what I am going to try first are a series of small anodes (in small vessels) using only the most basic reagents. I can always add nickel,
Bismuth, or surfactants later. I will focus on surface prep, and good control of temperature and current; hopefully the vibration + rotation will
create a dense, even plate.
Truly, the one thing I am a bit confused about is nitric acid evolution. How does one keep the acid concentration in check during a "one-pot" plating
effort? Too much acid = terrible grain and a poor plate.
The concentration of the nitric acid, and obviously the replenishment of the lead ions, as the PbO2 plates, are the major factors that need to be
controlled.
If the starting nitric acid concentration is 5 to 10 grams / liter, how do we keep it from climbing during the plate? Is it possible to simply hang
sheet Pb straps into the bath, and as the nitric evolves, the Pb dissolves? Or a dusting of litharge in the bottom of the vessel? A somewhat rough
equilibrium develops?
If the MMO plates successfully, the next step would be Ti round rod (drilled) or mesh. If a plating directly on a bare Ti anode is successful, that
would knock it. As you mentioned, Ti is easy to get these days. I am NOT overconfident. I know guys have been trying this for years. I just need
to get a feel for what is happening, and then hopefully, with a bit of group-think, we can solve the mysteries of PbO2 plate.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
A problem with hanging Lead metal sheet (or filings at the bottom) is that you will get the dreaded brown gas as it reacts with the Nitric acid. It
probably will not react with the acid at a concentration as low (6 - 10ml) as you are going to have in the tank anyways.
Litharge can be slowish to react with Nitric acid unlike Lead Carbonate or Basic Lead Carbonate which reacts much easier. Litharge gives no
fizzing/bubbles. How easy/quick the Litharge reacts depends on how fine it is and on how it was made. The fact that it does not react very
quickly/easy is an advantage as it will not eliminate all the Nitric acid but will only start reacting as the conc. of acid gets higher.
There is no easy way to keep the acid conc. at (say) 10 grams per liter without some sort of automatic pH control and some way of feeding Lead
compound into tank. I don't know if you could even use pH control as since the tank pH is going to be so low the pH will be very low.
What is the difference in the pH of a solution containing 10 grams per liter acid and 20 grams per liter acid. 0.90 versus 0.85??? Is it easy to
measure this with a cheapish pH meter? Perhaps someone that knows more about pH measuring can answer that.
You could make a slurry of Lead Carbonate for pumping into tank if that helps.
Some have suggested hanging a 'tea bag' of Litharge in tank.
Some sort of baffle hanging in the tank with (say) 3/4 of the tank where the anode is being plated and the other quarter where you add lots of Lead
compound. The two bodys of liquid will mix by diffusion. It's really similar(ish) to two tanks.
If the Lead Dioxide could be plated from a neurtal tank then it would be easy. Just add large amounts of Carbonate and the acid with never get up
above a few parts of grams per liter.
Add approx. 10 grams of Nitric to a liter of water and get an idea of how quick your Litharge will react. I have heard of guys getting it hard to get
the Litharge to dissolve at all!
You could perhaps ball mill it?
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I've got PbO aplenty, no lead carbonate. I like all the ideas behind a slow/steady "diffusion", or periodic additions of reagents. I will attempt to
produce lead carbonate from lead nitrate, since I have plenty of the latter, but none of the former.
That is a good thought, to do a bit of chemistry (experimentation) with nitric acid and various lead salts prior to plating, to get a feel for what is
likely to happen. I would KILL for the lab I worked in in the AF... we had every analytical instrument known at the time. Now it's back to basics...
literally, as 25 years away from chemistry has caused me to dump most of what I learned. My kids come for help with HS chem, and it takes me a while
before I can help. The knowledge is there, it's just buried, VERY deeply.
The patents are explicit - lead dioxide plates exceptionally well at low acid concentrations, and the plating suffers as the concentration climbs.
Perhaps something as crude as low-range pH paper and an appropriate additive to keep pH in check. I do have a pH meter but am leery of using the one
good probe I have in this bath. There will probably be gross interference anyhow to the pH meter from the plating current, requiring sampling of the
bath into a separate vessel.
Anyway, the initial experiments are going to be small. If a particular bath and methodology appears to work very well, then anodes sized approx. 12cm
x 4cm will be plated, as these are the correct size for my actual perc cell.
Roscoe, I am now the proud possessor of 1 ea kilogram of ultrapure gamma ALOOH, water-dispersible Boehmite alumina. Research/experiments to follow!

|
|
|
granitestaterecovery
Harmless

Posts: 26
Registered: 25-12-2008
Location: beside myself
Member Is Offline
Mood: combative
|
|
Swede may I ask what the optimum tempature of the plating bath and keeping it at a constant temp?I have a way to acheive this rather simply without
having to regulate your electrodes.
Granite State Recovery & Recycling
All our scars in life are self inflicted
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Hmmm...samples are always good.....but I think the gamma there would be Al2O3 ...unless they sent you some of the
unfired air dried precursor material which is the hydroxy form there. There is likely something useful to be gained with whisker alumina as a
"modifier oxide" reenforcing in these coatings I think especially the baked coatings and possibly even in the plated coatings. It sort of reminds me
of the
fiber reenforcing that wall plasterers and stuccoers used, horsehair in the olden days and later chopped fiberglass,
it's the same idea for the nano-whiskers of alumina.
If you are running a near neutral plating bath, to keep the pH constant will probably require a pumped electrolyte .....
with some kind of a power filter containing a neutralizing lead compound, like perhaps a finely divided lead oxide.
I think ideally to accomplish a steady state plating bath,
you would need a small secondary isolated AC electrolysis cell having two Pb plates , in the electrolyte recirculation loop
for the anode plating cell, and whatever lead was consumed
by the anode plating would be replenished by AC erosion
of the "Pb ion replenishment anodes" .....now that would be perfection huh ?  But a compromise arrangement could
But a compromise arrangement could
probably be gotten that would work good enough just using
a manual incremental neutralization and a generous sized
plating bath to dampen any wide swings in the cell chemistry
between the increments. If you know what rate of reaction
your plating current is producing , then you can calculate
the rate at which you need to add neutralizing material to
maintain the chemistry of the plating bath.
Hmmm....new years is not far away , and Valentine's Day
a bit around the corner still ...sooo , just being forward looking there at the possibilities , it is definitely time for a diva break  Whew this song is beautiful, a real wedding song if there ever was one. Enjoy. Whew this song is beautiful, a real wedding song if there ever was one. Enjoy.
http://www.youtube.com/watch?v=Dcy2zOsmK48&NR=1 (lyrics)
http://www.youtube.com/watch?v=LbtJR6sy3KE&fmt=18 (stereo and video) Wow !
Now I wouldn't mind some samples of that, even a lifetime supply, and I'm sure that's a popular sentiment among any men who still have a pulse 
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by granitestaterecovery
Swede may I ask what the optimum tempature of the plating bath and keeping it at a constant temp?I have a way to acheive this rather simply without
having to regulate your electrodes. |
If by regulating electrodes you mean using the plating process itself to heat the bath, that will not work... the current is too low.
Every source I've read specifies between 60 and 80, with one oddball source at 90 degrees. I'd split the difference and shoot for 70. I do not know
the effects, good or bad, of temperature variation on the quality of the plating. The patents also tend to specify +/- 1 degree C. I can't imagine
it's that critical.
Remember, I am at the theoretical stage now... I haven't begun to plate, just doing research. But I've got the hardware and chems ready to go. If
you have any insight to the process, welcome!
Roscoe: The supplied MSDS has the following:
Components:
Boehmite CAS 1318-23-6 95-97.5%
Water 2.5-5%
"Water-dispursable Boehmite Alumina, Aluminum oxide hydroxide, Gamma-ALOOH [crystallagraphic designation]"
[Edited on 27-12-2008 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
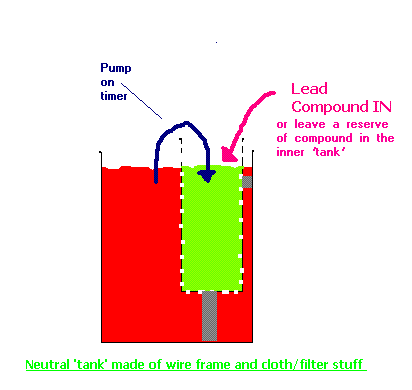
Perhaps the scheme like below would do for keeping Lead Ion replenished + neurtalize. It's getting close to two tanks as it were. You already have a
pump. The neutral solution will flow into plating bath proper as you pump liquid from plating bath into neutralizer.
There is plating equations on an article I attached (next post). It also states that anodes (ozone production) last longer if manufactured from a
plating tank that is warm. Lots of patents state that a 'better' plating is obtained from a warmed bath. I would agree that 70C is about right.
You will soon start to suffer from 'Article/patent fatigue', so watch out.
For every mole of Lead you get three moles of Nitric acid.
If plating efficiency goes low (say Nitrites build up) this may no longer apply. I don't know.
I do not think putting a pH meter into a plating tank liquid sample will harm it. But can you really monitor acid concentration by measuring pH?
People have always said that a new fresh plating tank always give the best coat (garage operators). All sorts of things were blamed. Nitrites perhaps?
One guy suggested bubbling air (O2) into tank between plating sessions. (You can use H202 of course).
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Attached is:
Electrosynthesis and Physicochemical Properties
of PbO2 Films
Journal of The Electrochemical Society, 149 ~9! C445-C449 ~2002!
Happy reading.
Attachment: alpha or beta for acid bath.pdf (94kB)
This file has been downloaded 951 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@Swede , you got the unfired material.....hmmm
that is fine, it should develop nicely to the dehydrated
form on baking. Did they send you a sample of the pyrolyzed form as well ?
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Rosco Bodine
@Swede , you got the unfired material.....hmmm
that is fine, it should develop nicely to the dehydrated
form on baking. Did they send you a sample of the pyrolyzed form as well ? |
Nope, for now just this stuff. They are also sending some material from Germany that is going to take some time to get here, but it is more of the
same, just different crystal and pore sizes, and perhaps acid-dispersing as opposed to water dispersing.
@Dann2, where do you come up with all this stuff?  I remember Tentacles had
troubles with his plating bath when it became a "used" plating bath. The implication is strong... if a one-pot anode is attempted, you just might get
one or two good anodes before the bath goes to hell, and the size of the bath determines how long it takes. I remember Tentacles had
troubles with his plating bath when it became a "used" plating bath. The implication is strong... if a one-pot anode is attempted, you just might get
one or two good anodes before the bath goes to hell, and the size of the bath determines how long it takes.
Lead Nitrate is selling for $10 kg, which will make about 3 liters of solution. That is a big bath for a garage lab, and should produce several good
anodes before becoming exhausted.
The initial thought is "Add lead nitrate on a molecular equivalence to the weight of the plated lead dioxide" but that doesn't help when the nitric
acid levels are climbing.
Traditional replenishment lead sources have been litharge (PbO) and Pb metal. Lead carbonate may be a superior replenishment material. Determine the
amount of lead nitrate lost, add that amount to a beaker, convert to the insoluble carbonate with Na carbonate or even bicarbonate, decant the bulk,
which will be sodium nitrate dissolved in water, and add the lead carbonate to the bath, which will be in the form of white lead:
2PbCO3·Pb(OH)2
I might be able to come up with an equation, but my gut feel is that excess nitric is neutralized, CO2 is gassed off, water forms, and lead nitrate
levels are restored.
Just a thought.
There are two paths we can go down... the first is to use up a bath until it no longer plates worth a damn, the second is careful maintenance of the
bath no matter how large. Some sort of circulation setup is tempting, but I think the best results would come from a medium to large bath plating
modest electrodes. At the end of the plating session, the anode is weighed, and an appropriate amount of lead (in whatever form) is returned to the
bath. If the bath is large to start with, changes to the chemistry of the bath DURING plating will be small, and hopefully be optimum thoughout the
given plating session. The small changes that take place during plating are then "repaired" with lead carbonate or litharge after the session.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
On a totally different note, has anyone heard of pulsing a plating bath to produce a superior plate? This would be extremely easy to do with a
half-wave rectifier on a variable AC transformer. You'd get a half-sinusoid - no reverse current flow, but the forward flow would pulse ON and OFF at
the mains frequency. This may in fact allow the bath to "re-wet" the anode, especially if vibrated or rotated, between positive pulses, and
microscopic gas bubbles may have a chance to escape between pulses. The overall result may be a smoother, better adhering coat of lead dioxide.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by Swede
On a totally different note, has anyone heard of pulsing a plating bath to produce a superior plate? |
Never tried it on plating, but I have tried it on electrolytic
reductions, and it works supposedly better for some reactions than smooth DC. It will make an audible power hum in the cell at high currents...so you
won't need any vibrator at high currents..the cell itself becomes one 
Of course that is sort of the extreme in terms of current density 
| Quote: |
This would be extremely easy to do with a half-wave rectifier on a variable AC transformer. You'd get a half-sinusoid - no reverse current flow,
but the forward flow would pulse ON and OFF at the mains frequency. This may in fact allow the bath to "re-wet" the anode, especially if vibrated or
rotated, between positive pulses, and microscopic gas bubbles may have a chance to escape between pulses. The overall result may be a smoother,
better adhering coat of lead dioxide. |
Yeah it is a special technique which can be used but I'm not sure how to predict the effect, it is purely experimental.
You can use phase control to shape the delivered waveform
to an assymetrical AC with whatever pulse sequence you
want to send the cell......sort of like sending it Morse code
in power pulses , hoping it will get the "telegram" of what you want it to do. If it is not cooperating ....hey just increase
the power until something breaks  
The power company really loves you for what sort of untypical and unpredictable noise loads this imposes on the grid, it really makes every
transformer along the road to your house "sing for joy" over the experiments you are doing, and might elicit a visit from the grid maintenance people
to see what in the hell you are doing 
For a neutralizer you might want to look at basic lead hydroxide, but it is temperature sensitive and above 45C it
converts to the normal oxide. Basic carbonate would be good too possibly better for a hot electrolyte, but you really do need a pumped electrolyte so
you can allow for the effervescence of the CO2 byproduct to occur in a separate neutralizing tank which is a low velocity flow rate reservoir. Really
there is a need for using pumped flow on all of these processes for various physical considerations, and I think when this is all over, pumped flow
electrolytes and coaxial electrode assemblies is inevitable......for plating or for perchlorate. Tanks will become reservoirs, but all the
electrolysis will occur inside the flow through electrode assembly.
[Edited on 28-12-2008 by Rosco Bodine]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I have never heard of Lead Metal being used to replenish Lead Ions but I see no reason why not to use it. It will not react too easily with the lowish
concs. of Nitric that you want to try and work at.
The last paper I posted was from the 'More on PbO2 anodes' thread. Lots lots more there is you want to go digging.
There is some more stuff on observations of Lead Compounds that have been used to replenish/nutralize the plating tanks here:
http://www.geocities.com/CapeCanaveral/Campus/5361/chlorate/...
See 'looking after the plating tank'. You have probably seen it before.
There is an attached patent for an anode that uses AC on DC. It uses the (what I call anyways) high Nitric acid plating tank. (100grams per liter
acid). It is an electrowinning anode on bare!!!!!!!!!! Ti.
The title in incorrect, it should say Anode not Cathode.
I would suggest sticking to good old DC.
Dann2
Attachment: US4159231.pdf (315kB)
This file has been downloaded 695 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I think there may be a scheme for using very low voltage AC on two plain lead electrodes, to supply replacement lead ion to a plating electrolyte at
a similar rate as lead is being plated out by DC onto a target anode. There are patents related to the production of lead salts by electrolysis, but
I don't recall them being specifically described in a scheme for maintaining the cell chemistry for a plating bath. However it may be possible to do
that, can't see any reason why not. The idea is that the low voltage AC would have just enough potential to defeat the oxide passivation layer on the
lead and cause it to be easily corrodable by any acid present going into solution as the salt of that acid, from reaction with the
nascent lead hydroxide or even the lead metal.
The idea there is to apply just sufficient potential to reduce the natural oxide film which is passivating,
but not have a high enough voltage to produce an
immunity to corrosion by hydrogen blanketing.
The Pourbaix diagram for lead if I recall correctly said something like three quarters of a volt cathodization potential is about all it takes to
depolarize lead for active corrosion. You could probably use DC also for the same purpose. The circuit here for the "neutralizer cell" would have to
be electrically isolated on a floating independent source apart from the power supply to the anode plating scheme. The way I think something like
this may work is using parallel sheets of lead having strips of insulating spacers which act as separators between the plates, wound into a spiral
coil form cylinder and housed in a
PVC pipe, through which the connection is made to riser tabs from the lead sheet electrode pair inside. The pumped electrolyte enters the bottom of
the housing
and fills the housing as it rises inside, wetting the spirally
oriented sheet electrodes up to about three quarters of their height , encountering perforations in the plates and
exiting through those perforations through disharge
openings on the sides of the housing, as a level regulating overflow means. The discharging neutralized electrolyte would then pass through a gravity
filter and
discharge back into the reservoir for the plating cell.
If a pumped plating cell was being used, a portion of the flow could simply be diverted through the neutralizer.
|
|
|
granitestaterecovery
Harmless

Posts: 26
Registered: 25-12-2008
Location: beside myself
Member Is Offline
Mood: combative
|
|
swede i was reffering to you regulated heating method of the electrodes with electric ceramic inserts.I have proven that they have short life span.
. I sent an u2 or whatever the messaging language is to your profile about it ....but it may be bad for both electrodes /anode and diode to become
positive due to malfunctioning electric heaters within the unforeseen reason for your neighborhood to be evacuated.... Some people just dot understand
the quest for knowledge...or things they find when evacuating..
Granite State Recovery & Recycling
All our scars in life are self inflicted
|
|
|
| Pages:
1
..
15
16
17
18
19
..
48 |
|