phenol
Aniline is one of the most useful organic intermediates since with diazotization it can be readily converted to a benzene ring having one of a wide
range of functional groups. Those who have completed a course in organic chemistry already know this.
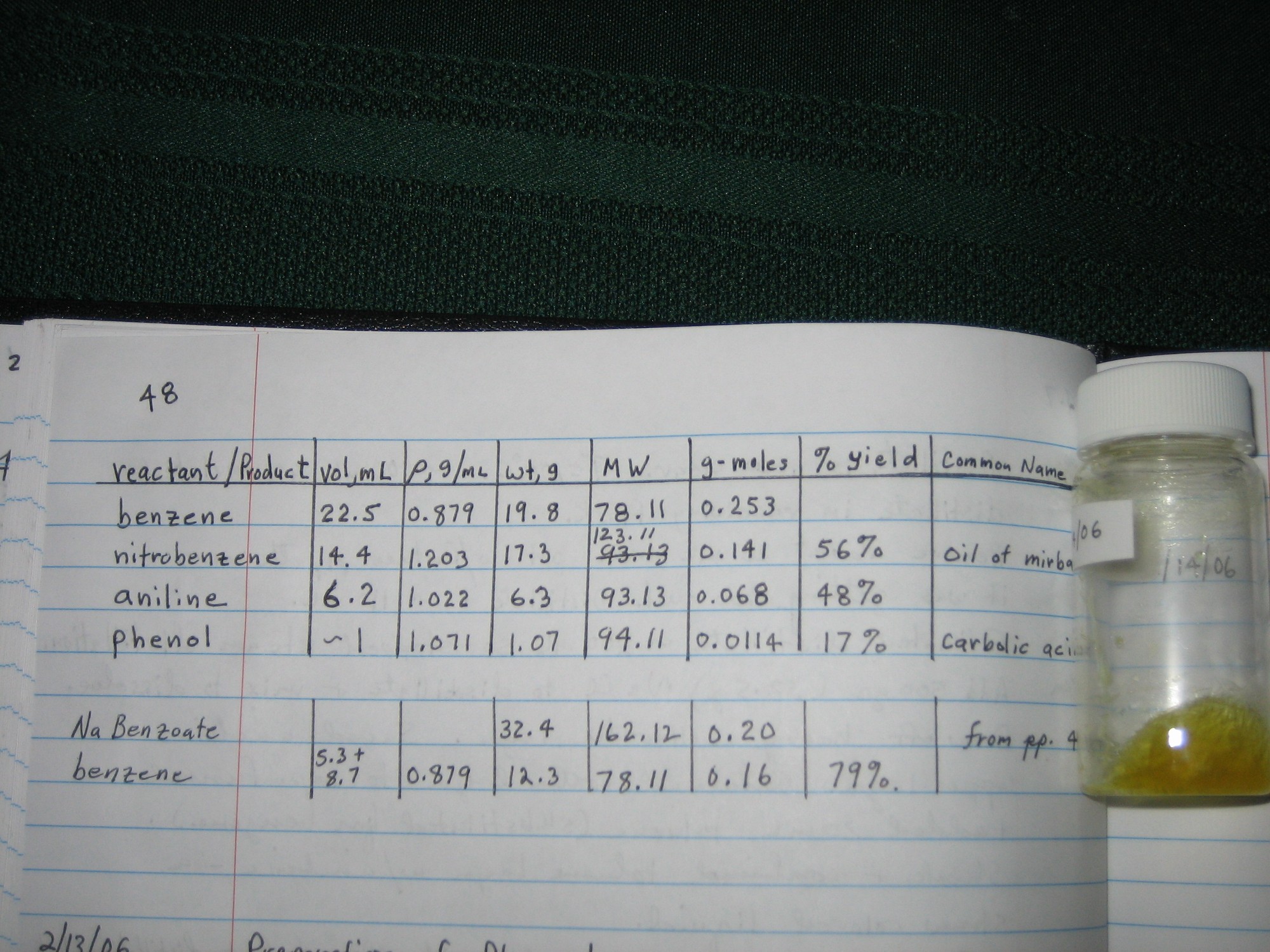
Of the many options, I chose to convert my small amount of aniline to phenol. The attached photo shows the phenol, the synthetic route, and the yield
of each step. These yields aren't very good so please don't take them as representative. I blame the poor yields on the fact that high surface to
volume ratios were causing ever increasing losses. But as someone else has indicated "one can always find an excuse for a poor yield." 
Edit: FYI I assumed my Na Benzoate was hydrated with 1 molecule of water.
[Edited on 15-2-2006 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|