careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Vilsmeier-Haack Reaction
I've been reading up on the Vilsmeier-Haack reaction (there are a couple of surveys of the subject on-line) and was impressed by the wide variety of
choices there seem to be for both reactants used to prepare the Vilsmeier reagent.
http://www.ijpcbs.com/files/04-37.pdf
http://shodhganga.inflibnet.ac.in/bitstream/10603/125/6/6_ch...
For the amide:
Dimethylformamide, N-Methylformamide, N,N-Dimethylformanilide, N-Methylformanilide, N-Methyl-2-pyrrolidone, N-Methylacetamide, Dimethylacetamide,
N-methylacetanilide, (ignoring many others that had various caveats expressed)
For the acid:
phosphoryl chloride, thionyl chloride, phosgene, oxalyl chloride, phosphorus pentachloride, etc.
Mostly people seem to use DMF and POCl3, but other combinations are not hard to find in the literature. Is there a better review of this subject
anyone can point me to where selection issues (if any) among all these reagents are discussed?
If it is purely a matter of convenience/cost among these choices it would seem that DMF and thionyl chloride or oxalyl chloride (all available from
Elemental Scientific now) would be obvious choices for SM members, avoiding the need to prepare POCl3 (though that sounds like an interesting project
on its own).
I suspect there are other factors involved that were simply not discussed in the write-ups I perused.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by careysub  | For the amide:
Dimethylformamide, N-Methylformamide, N,N-Dimethylformanilide, N-Methylformanilide, N-Methyl-2-pyrrolidone, N-Methylacetamide, Dimethylacetamide,
N-methylacetanilide, (ignoring many others that had various caveats expressed) |
You cannot just chose any such amide. It depends on what kind of an acylation you want. If you are doing a formylation, you need to use formamides
(DMF, N-methlyformanilide, etc., and BTW, there is no such thing as "N,N-Dimethylformanilide"). If you use acetamides you get the acetylation
products, and so on. See the reaction mechanism.
| Quote: |
For the acid:phosphoryl chloride, thionyl chloride, phosgene, oxalyl chloride, phosphorus pentachloride, etc. |
Those are not acids. They are acid chlorides. But it does not have to be acid chlorides either. It can be anything that can form the Vilsmeier reagent
in situ. Equivalently reactive R2N+=CXR reagents formed in situ, where X is a group with a strong enough -I
effect also work. In principle, even acetic anhydride with DMF can be used for the formylation provided the substrate is reactive enough (but good
luck finding one). Oxalyl chloride is the ideal activating reagent as it give the Vilsmeier reagent cleanly and quantitatively, with just
CO2 and CO as side products. POCl3 is however just fine and most practical.
| Quote: | | Mostly people seem to use DMF and POCl3, but other combinations are not hard to find in the literature. Is there a better review of this subject
anyone can point me to where selection issues (if any) among all these reagents are discussed? |
How about the reviews posted in other threads on the subject?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by Nicodem  |
You cannot just chose any such amide. It depends on what kind of an acylation you want. If you are doing a formylation, you need to use formamides
(DMF, N-methlyformanilide, etc., and BTW, there is no such thing as "N,N-Dimethylformanilide"). |
Editing error - the review cited "N-dimethylformanilide". Sorry for the extra "N".
| Quote: | | If you use acetamides you get the acetylation products, and so on. See the reaction mechanism. |
Should have dropped the acetamides from the list. I should know better than post late at night.
| Quote: |
Those are not acids. They are acid chlorides. |
Thanks, I knew that. (Again that late night posting thing.)
| Quote: | | But it does not have to be acid chlorides either. It can be anything that can form the Vilsmeier reagent in situ. Equivalently reactive
R2N+=CXR reagents formed in situ, where X is a group with a strong enough -I effect also work. In principle, even
acetic anhydride with DMF can be used for the formylation provided the substrate is reactive enough (but good luck finding one). Oxalyl chloride is
the ideal activating reagent as it give the Vilsmeier reagent cleanly and quantitatively, with just CO2 and CO as side products.
POCl3 is however just fine and most practical. |
Excellent! This is the sort of information I was looking for. Thanks!
| Quote: | | Quote: | | Mostly people seem to use DMF and POCl3, but other combinations are not hard to find in the literature. Is there a better review of this subject
anyone can point me to where selection issues (if any) among all these reagents are discussed? |
How about the reviews posted in other threads on the subject? |
I didn't list them, but I did read the top 15 or so threads that came up for the SM site using the keyword "Vilsmeier". According to Google there are
"about 75" hits for this. I'll work on down the list to see if there are other reviews linked.
[Edited on 8-12-2014 by careysub]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
cobalt chloride may be substituted for POCl3
if you want to make only aryl aldehydes and not aryl ketones (Vack can do both) ,dont you think the duff reaction is much easier
considering that hexamine is easier to make than instant coffee 
http://en.wikipedia.org/wiki/Duff_reaction
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
Interesting indeed. Please attach the paper; or point towards the paper.
[Edited on 9-12-2014 by forgottenpassword]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
2nd page ,under "Reagents"
http://www.ijpcbs.com/files/04-37.pdf
also read the third post by Magic muzzlet
http://www.sciencemadness.org/talk/viewthread.php?tid=21927
it seems even HCN can be used 
http://chemistry.mdma.ch/hiveboard/methods/000372127.html
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
http://en.wikipedia.org/wiki/Phosgene

|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
CoCl2=cobalt chloride
COCl2=phosgene
HCN is used in the Gatterman formylation, rather than Vilsmeier.
AFAIK, Duff formylation only works on fairly well activated substrates, Vilsmeier can be used for the less activated ones.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Yes, the Gatterman synthesis. It can produce quite high yields.
I could not find a good write up on the Gatterman aldehyde reaction online, so I bought an old copy of Organic Reactions (see below) for a few bucks.
The survey article is very useful. I intend to scan it and contribute it to the library (and maybe Scribd).
Most people use zinc cyanide these days (Roger Adams developed this technique) which is prepared or obtained without handling liquid HCN.
Adams, Roger (1957). Organic Reactions, Volume 9. New York: John Wiley & Sons, Inc. pp. 53–54.
I was looking for one of similar quality for Vilsmeier-Haack.
|
|
|
Boffis
International Hazard
    
Posts: 1838
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
The relevant review for the Vilsmeier Haack reaction is:
Organic Reactions vol 49 1996 pp1-132.
I am not at home at present so I can't access my hard copy but the chapter on this reaction is c 130 pages and give several examples, excellent. I am
interested in a slight modification of this reaction to produce 2-chloroquinolines and related compounds. From this compound I hope to produce
2-quinolinehydrazine.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
forget POCl3 or any other chemical
even nicodem (who always gives the best ideas) is going to be shocked 
i almost fell off my chair 
http://www.chem.wisc.edu/areas/organic/index-chem.htm
click "named reactions-smith" under the heading chemical reactions on the top left of the page and see the 88th named reaction-Vilsmeier Formylation
maybe microwaving will give even better yields and reduce reaction time ?
[Edited on 13-12-2014 by CuReUS]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by CuReUS  | forget POCl3 or any other chemical
even nicodem (who always gives the best ideas) is going to be shocked 
i almost fell off my chair 
http://www.chem.wisc.edu/areas/organic/index-chem.htm
click "named reactions-smith" under the heading chemical reactions on the top left of the page and see the 88th named reaction-Vilsmeier Formylation
maybe microwaving will give even better yields and reduce reaction time ?
[Edited on 13-12-2014 by CuReUS] |
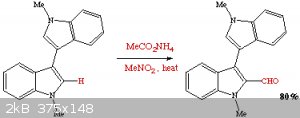
Since external sites are transient in their content (with few exceptions) here is the essential content for SM posterity:
Bergman, J.;* Desarbre, E. SynLett, 1997, 603-605
The title and full author ID is:
"Synthesis of Indolo[2,3-c]carbazole Derivatives by Thermal Electrocyclic Reactions" by Jan Bergman and Eric Desarbre.
Perhaps someone could access that.
Yes, Nicodem is - to use the French (not English) word - formidable.
[Edited on 13-12-2014 by careysub]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by careysub  | Since external sites are transient in their content (with few exceptions) here is the essential content for SM posterity:
Bergman, J.;* Desarbre, E. SynLett, 1997, 603-605
The title and full author ID is:
"Synthesis of Indolo[2,3-c]carbazole Derivatives by Thermal Electrocyclic Reactions" by Jan Bergman and Eric Desarbre. |
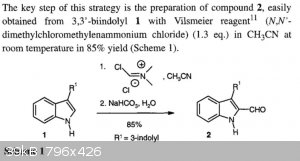
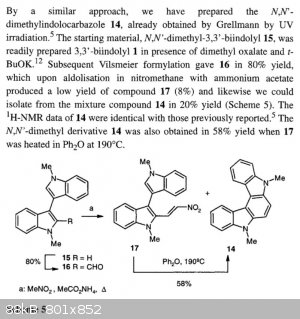
There is nothing special in that example, except in that they use the isolated Vilsmeier reagent in acetonitrile for the formylation of the bisindole,
rather than employing the Vilsmeier-Haack formylation method with the in situ reagent formation (the Vilsmeier reagent is commercially available). I
don't understand why CuReUS almost fell off his chair. Perhaps that near accident is unrelated to the above reference.
It is perhaps of interest in the view of another forum topic that they do the N,N'-dimethylbisindole by the methylation with dimethyl oxalate.
| Quote: | | Editing error - the review cited "N-dimethylformanilide". Sorry for the extra "N". |
It was not about an extra N, it is about an extra methyl. A formanilide can not have two methyls on the nitrogen and still be an amide.
[Edited on 13/12/2014 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
i didnt mean that.I thought that nicodem,who always comes up with the most beautiful yet mind boggling and counter intuitive ideas would be taken
aback by the sheer simplicity of the reaction
anyways, after seeing that the Vack reagent was used on the side,the reaction seems to have lost its charm 
btw, nicodem how did you get the paper so easily ,i googled it but some chinese nonsense came up
did you have the paper already ,or is it from some special website ?
[Edited on 14-12-2014 by CuReUS]
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
It's the journal 'synlett'. How would ANY chinese crap come up?! Go to their website and type in the volume and page number.
|
|
|