nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Phenoxyacetic Acid Synthesis
Hello all. Recently I have been looking into synthesizing 2,4-Dichlorophenoxyacetic acid to be used as a plant hormone in plant culture. At first I
though I would need to chlorinate phenol and react that with chloroacetic acid in order to produce the hormone, which is the route used industrially,
but than I found this paper with a much more convenient method that first creates Phenoxyacetic acid and chlorinates it with NaClO.
(1st synthesis) http://www.inchem.org/documents/ehc/ehc/ehc29.htm
(2nd synthesis) http://pubs.acs.org/doi/abs/10.1021/ed025p514?journalCode=jc...
Although this is much easier and safer than the first synthesis, it would be difficult for me to obtain chloroacetic acid. In addition, chloroacetic
acid is relatively expensive, and out of my budget for this project. Would it be possible to react Diglycolic Acid with Phenol to produce
Phenoxyacetic acid, similarly to how acetic anhydride is reacted with salicylic acid to produce acetylsalicylic acid? I realize diglycolic acid is not
an anhydride, but it's structure seems very similar. If it was possible to use Diglycolic acid for this synthesis, I plan on creating it through the
oxidation of Diethylene Glycol.
(Diglycolic Acid synthesis) http://pubs.acs.org/doi/abs/10.1021/i260075a013
Thank you,
Nick Legaux
[Edited on 6-17-2015 by nlegaux]
|
|
|
Pumukli
National Hazard
   
Posts: 724
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
You will probably need very small ammount of this stuff if you intend to use it as a plant hormone.
2,4-D is very potent, e.g. in tissue cultures the usual dose is around 0.2 - 0.5 mg/l if memory serves well! It is used as an artificial auxine-like
hormone but its main drawback afaik is that it induces callus growth and later nothing "plant-like" will emerge from that tissue blob.
What if you tried to purify it from a weed killer instead of the synthesis? (2,4D amine e.g., it is the dimethylamine salt of
dichlorophenoxy-acetic-acid)
Diglycolic acid would take you nowhere. You would need alpha-halo-acetic acid for the synthesis, making Aspirin is not a good analogue of this
reaction.
Ask your friends, neighbours, gardener if they can give you a few milliliters of an appropriate weed killer and you may have it sooner than you
thought at first!
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
I may be misunderstanding what you're suggesting, but I think you're confusing a few issues in your suggestion to use diglycolic acid. Let me
illustrate... I've left out details of the reagents for simplicity.
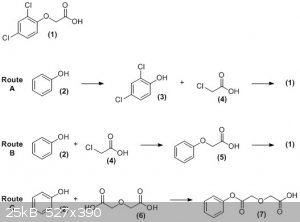
In the figure below, compound (1) is the target, 2,4-dichlorophenoxyacetic acid. You have found two routes by which it can be prepared - chlorination
of phenol (2), followed by alkylation of the dichlorophenol (3) with chloroacetic acid (4) (route A); or alkylation of phenol with chloroacetic acid,
followed by chlorination of the resulting phenoxyacetic acid (5) (route B). Both of these would get you to the desired product.
Now, as for the diglycolic acid (6) method (route C). Firstly, diglycolic acid is an ether, and is thus quite unreactive. Certainly it will not
alkylate a phenol under any reasonable conditions. The best you could hope for would be to acylate the phenol, analagous to the reaction used to
synthesise aspirin (although, again, this would require some other reagent to activate the diglycolic acid), and would give you the entirely unwanted
product (7).
Pumukli's suggestion to search for a commercial product from which you can extract it is a good one; another alternative would be to simply purchase
phenoxyacetic acid - if you have a source from which you could obtain chloroacetic acid, then phenoxyacetic acid should be likewise available (and a
lot cheaper).
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Thank you for the excellent suggestion Pumukli, tomorrow I plan on going to the local hardware store to see what herbicides they have in stock (I only
have Raid, whose MSDS doesn't list 2,4-D). I looked into purchasing Phenoxyacetic acid as a precursor, but I have been unable to find it for sale
beyond Sigma Aldrich. I will report on how I separate the 2,4-D from the herbicide.
|
|
|
AvBaeyer
National Hazard
   
Posts: 659
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
nlegaux,
I do not know how deeply you wish to get involved in synthetic chemistry, but phenoxyethanol is an easily available and relatively inexpensive
compound. This could be oxidized to phenoxyacetic acid then chlorinated.
Unfortunately, I just checked my go-to supplier and it is currently out of stock. However, an internet search should turn it up as phenoxyethanol is a
common ingredient in the cosmetics area.
AvB
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
Try aksci.com; very friendly and helpful supplier to me in the past (as an international customer, too), and they claim to have it in stock ($42 for
500g).
It's a very common chemical. If you like, I'd be happy to hit SciFinder and do a quick supplier search for you, but any respectable supplier should
have it or be able to get it.
Of course, as I noted, if you just want the product, try extracting; I only offer this in case you're actually interested in doing the chemistry!
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
For now I am only interested in obtaining the product, but in the future I would like to attempt synthesizing plant hormones in order to practice some
organic chemistry (if I don't apply it, there is no way I will remember it or get better at it). It is good to know that there is a supplier that
offers such a wide variety of organic compounds for a good price. I purchased some herbicide, "Spectracide Weed Stop for Lawns Concentrate", which is
listed as containing 7.59% 2,4-D (diethylamine salt), 1.83% Mecoprop-p (diethylamine salt), and 0.84% Dicamba (also diethylamine salt). Currently I
plan on separating the 2,4-D and Mecoprop-p from the Dicamba by liberating the acids using HCl. I believe this will precipitate the 2,4-D and
Mecoprop-p, whose solubilities are fairly low (900mg/L in water). At this point I'm not sure on how to separate the 2,4-D from the Mecoprop-p... Any
suggestions?
[Edit] I just attempted the separation. On addition of 6ml 10M HCl to 100ml of herbicide, a white precipitate formed along with a brown liquid
(diethylamine?).The precipitate was too fine to filter, even with my vacuum filtration setup, but I was able to decant the suspension off the top of
the brown liquid. I than attempted to recrystallize the precipitate by dissolving it (still in suspension) in 20ml acetone and adding water until it
recrystallizes (hopefully with larger crystals). Unfortunately, I didn't get a precipitate, even when 1L of water was added.
[Edited on 6-18-2015 by nlegaux]
|
|
|
Pumukli
National Hazard
   
Posts: 724
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Purifying something from a pesticide formulation is not easy. You expect things based on solubility data. Which is based on sulibilities of pure
materials in distilled water (most probably).
But you have 10% active ingredients and 90% something. This something contains a fair amount of surfactants and other ingredients (perhaps foaming
inhibitors, etc.) and these are not listed on the package of course. The solubility of a given compound in dest water and in a detergent bath is not
the same!
Sometimes boiling the freshly formed fine crystal precipitate for a few minutes would "age" it: bigger and more easily filtered crystals could
aggregate from it.
I would also check the solubilities of the required compound in several different solvents, maybe acetone is not the best choice in your case, I don't
know.
Keep us informed, I'm especially interested in the outcome of your experiment!
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I always wondered what would happen if glycine was reacted with HNO2.
if it formed 2-hydroxyacetic acid,you could easily convert that to chloroacetic acid(Lucas reagent).I also remembered yesterday that there was a
procedure for making chloracetic acid is the "War gases" book.(using Sulfur instead of phosphorous)
| Quote: | | It is possible to substitute sulfur for red phosphorus in this reaction, which is much more readily available, it is not as efficient as phosphorus
though. |
https://www.erowid.org/archive/rhodium/chemistry/chloroaceti...(under method B[1])It was also found out quite recently that you could make
chloracetic acid from acetic acid,Cl2 and acetyl chloride.
another way to get 2-hydroxyacetic acid would be to do an intramolecular cannizaro on glyoxal.
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Earlier I checked the MSDS for the product and nothing else was listed, but as you said Pumukli there is probably a great number of other ingredients
in the herbicide. Does anyone have any ideas on how to remove or identify them?
Today I attempted the separation a second time, with a few changes. I began by reacting 100ml of herbicide with 6ml of 10M HCl. As before, a white
precipitate immediately formed and a brown liquid settled on the bottom. I decanted the suspension off the top and heated it for 10 minutes, after
which the suspension had turned clear (with a brown tint to it). I than put it inside a refrigerator at about 4 Celsius for an hour. After the hour
the precipitate had reformed (still too fine to filter) and there was a small layer of brown liquid at the bottom. I decanted the suspension off the
top, and heated it until it was clear (now with no brown tint). I'm now allowing it to cool off at room temperature to (hopefully) allow crystals to
form large enough to filter.
That is a much easier way to synthesize chloroacetic acid CuReUS... If I am able to get the materials I will have to try it 
|
|
|
Pumukli
National Hazard
   
Posts: 724
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
There is an even simpler synthesis, CuReUS, but your original idea about HNO2 was right!
I almost fear to mention it again, but that Hilgetag-Martini-Weygand book (Preparative Organic Chemistry, available in the SM library) what I read and
reread when I have some time knows the answer to this question as well.
Actually there is an entire sub-chapter dedicated to it. (page 256, sub-chapter "c", titled: Replacement of NH2 in aliphatic compounds by
halogen by means of a nitrosyl halide)
I won't retype the entire sub-chapter here (although it is less than a page long) but it basically says that aliphatic primary amines and amino
carboxylic acids give halides (and not diazonium compounds) when treated with NOX:
R-NH2 + NOX -> RX + N2 + H2O
In practice the NOX is generated in situ from HNO2 plus halo-acid or halogenide-salt plus sulfuric acid. It seems that bromides give much
better yield than chlorides though. (At least this is the case with alanine -> 2-halo-propionic acid.)
Relevant articles:
P. Walden, Ber.Deut.Chem.Soc., 28, 2766 (1895) and 29, 133 (1896)
|
|
|
AvBaeyer
National Hazard
   
Posts: 659
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
The conversion of alpha-amino acids to alpha-bromo acids with NaNO2/HBr (or KBr/H2SO4 to generate HBr in situ) is a well established method. I believe
this has been published in Organic Syntheses but I cannot immediately find the reference. I have run these reactions many times with all sorts of
amino acids - some work better than others. Glycine is amongst the worst performers probably because bromoacetic acid is hard to isolate from the
reaction mixture. The reaction is very useful stereochemical reasons. If you start with an optically active amino acid, the reaction proceeds with
retention of configuration because the intermediate is an alpha-lactone.
AvB
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
The precipitate formed after allowing the solution to cool at room temperature is still too fine to be filtered and does not settle, which leads me to
believe that the product contains a dispersant/surfactant of some kind. Does anyone have any suggestions for removing the surfactant?
[Edit] After doing some research and double checking all of my facts, I have discovered that I misread the label. It is the dimethylamine
salt, not the diethylamine salt. I apologize for this oversight on my part. According to the information I have been able to find on the
material, it is an amber-colored liquid.
http://npic.orst.edu/factsheets/2,4-DTech.pdf
This leads me to believe that the amber colored liquid I have been discarding may be the 2,4-D (dimethylamine salt).
[Edited on 6-20-2015 by nlegaux]
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
Because of the new information stated previously, I have attempted the separation a third time. I started by reacting 50ml of herbicide with 3ml of
10M HCl. I than decanted off the suspension, leaving the brown liquid. I than reacted the brown liquid with 2g NaOH (previously dissolved in 30ml
water) with stirring. The brown liquid dissolved into the NaOH solution, and after about 2 seconds a white gelatinous precipitate formed. Next, I
filtered this precipitate and washed it. I found that when washed, it dissolves with some foaming. Once the precipitate dries, I plan on reacting it
with HCl. This is because I believe it to be the sodium salt of 2,4-D and mecoprop-p. Assuming a precipitate forms after addition of HCl, how could I
test to see it is 2,4-D, beyond determining it's melting point?
[Edit] When HCl is added to what I believe to be the sodium salt of 2,4-D and mecoprop-p, a white insoluble precipitate forms. I repeated the same
procedure with 200ml of herbicide with the same results.
[Edited on 6-21-2015 by nlegaux]
|
|
|
Pumukli
National Hazard
   
Posts: 724
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Is 2,4-D an amber colored liquid?
I have seen a small vial in a laboratory somewhere with a sticker "2,4-D" and a whitish grey powder was in the vial. Dimethylamine salt of 2,4-D as a
liquid? It is a surprise for me.
|
|
|
nlegaux
Hazard to Self
 
Posts: 93
Registered: 28-11-2014
Location: East Tennessee
Member Is Offline
Mood: No Mood
|
|
On the MSDS the dimethylamine salt of 2,4-D is listed as an amber colored liquid. Whether this means it is typically sold in solution or the salt is a
liquid at room temperature I'm not sure, because I haven't been able to find much information on this salt beyond it's environmental effects.
nlegaux
|
|
|
Dr.Bob
International Hazard
    
Posts: 2893
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
The pure 2,4 acid would be a solid, but many of those plant hormones are used as impure mixtures, thus why Agent Orange was contaminated with
dioxanes. Now they purify them enough to remove the dioxanes and other nasties down to below ppm, but the acid is still not 100% pure. Plus the
dimethylamino salts of those acids are very hygroscopic, so even if pure, they will dissolve themselves in their own water of hydration, in most
cases. Most are handled as aqueous solutions and never dried.
|
|
|