Synthesis of 1-hydroxypyrene-tris-3,6,8-octadecyl sulfonamide
Synthesis of 1-hydroxy-pyrene-tris-3,6,8-octadecylsulfonamide, a lipophilic derivative of the pH indicator
trisodium-1-hydroxypyrene-3,6,8-trisulfonate. The derivative can be immobilized in a gel or plastic matrix and used in various sensors such as the one
presented in Larsen et al (2011); look at Kühl (2005) for an introduction to various optical sensors.
The synthesis is basically a scaled down version of the one you find in Mohr et al (1995).
Materials
Trisodium-1-hydroxypyrene-3,6,8-trisulfonate, sodium acetate, acetic anhydride, methanol, phosphorus pentachloride, toluene, dimethylformamide,
octadecylamine, sodium hydroxide, distilled water. All reagents used are reagent grade. Some aren't easy to acquire for the hobby chemist, but perhaps
someone who has access to them fancy trying it out... otherwise people can enjoy my questionable photography skills.
Reaction overview
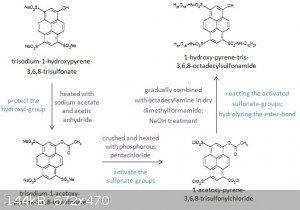
Procedure
905 mg (1.76 mmol) of trisodium-1-hydroxypyrene-3,6,8-trisulfonate, 174 mg (1.79 mmol) of sodium acetate, and 5 ml of acetic anhydride were stirred
at 60⁰C for 150 min.

The resulting mixture was filtered and the liquid evaporated to dryness, revealing an opaque yellow residue.

The crude product was recrystallized using 80 ml methanol.

The recrystallized material was thenfiltered and evaporated to dryness. Yield: 505 mg (51%) trisodium-1-acetoxy-pyrene-3,6,8-trisulfonate.
The 505 mg (0.894 mmol) of trisodium-1-acetoxy-pyrene-3,6,8-trisulfonate was ground in a mortar with 1820 mg of phosphorus pentachloride and the
mixture was heated at 100⁰C for 30 min. At this point 50 ml toluene was added and the mixture was stirred for 20 min and was then filtered and
evaporated to dryness. Yield: 381 mg (77%) of 1-acetoxy-pyrene-3,6,8-trisulfonyl chloride.
381 mg (0.686 mmol) of 1-acetoxy-pyrene-3,6,8-trisulfonyl chloride is dissolved in 20 ml of dry dimethylformamide. This solution is slowly added to
569 mg (2.11 mmol) of octadecylamine in 40 ml of dry dimethylformamide.

The resulting mixture is stirred for 80 min and then heated to 90⁰C for 15 min. The O-acetyl ester bond is then hydrolyzed by treatment with 0.1M
NaOH for 30 minutes. After cooling, the product is precipitated with distilled water.

The precipitate is then filtered, washed with methanol and dried. Yield: 683 mg (82%) of 1-hydroxy-pyrene-tris-3,6,8-octadecylsulfonamide.
Here the product has been dissolved in 3ml chloroform for later use.

References
Larsen et al (2011) Limnol. Oceanogr.: Methods, 9: 348–360
Kühl (2005) Methods in enzymology, 397: 166–199.
Mohr et al (1995) Journal of Fluorescence, Vol. 5, No. 2: 135-138
[Edited on 13-10-2015 by Einherje]
|